Cyclin-C-dependent cell-cycle entry is required for activation of non-homologous end joining DNA repair in postmitotic neurons
- PMID: 20111042
- PMCID: PMC2885568
- DOI: 10.1038/cdd.2009.221
Cyclin-C-dependent cell-cycle entry is required for activation of non-homologous end joining DNA repair in postmitotic neurons
Abstract
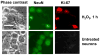
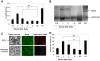
It is commonly believed that neurons remain in G(0) phase of the cell cycle indefinitely. Cell-cycle re-entry, however, is known to contribute to neuronal apoptosis. Moreover, recent evidence demonstrates the expression of cell-cycle proteins in differentiated neurons under physiological conditions. The functional roles of such expression remain unclear. Since DNA repair is generally attenuated by differentiation in most cell types, the cell-cycle-associated events in postmitotic cells may reflect the need to re-enter the cell cycle to activate DNA repair. We show that cyclin-C-directed, pRb-dependent G(0) exit activates the non-homologous end joining pathway of DNA repair (NHEJ) in postmitotic neurons. Using RNA interference, we found that abrogation of cyclin-C-mediated exit from G(0) compromised DNA repair but did not initiate apoptosis. Forced G(1) entry combined with prevention of G(1) --> S progression triggered NHEJ activation even in the absence of DNA lesions, but did not induce apoptosis in contrast to unrestricted progression through G(1) --> S. We conclude that G(0) --> G(1) transition is functionally significant for NHEJ repair in postmitotic neurons. These findings reveal the importance of cell-cycle activation for controlling both DNA repair and apoptosis in postmitotic neurons, and underline the particular role of G(1) --> S progression in apoptotic signaling, providing new insights into the mechanisms of DNA damage response (DDR) in postmitotic neurons.
Figures





Similar articles
-
Cell cycle activation in postmitotic neurons is essential for DNA repair.Cell Cycle. 2007 Feb 1;6(3):318-29. doi: 10.4161/cc.6.3.3752. Epub 2007 Feb 27. Cell Cycle. 2007. PMID: 17297309
-
Context-Dependent Functions of E2F1: Cell Cycle, Cell Death, and DNA Damage Repair in Cortical Neurons.Mol Neurobiol. 2020 May;57(5):2377-2390. doi: 10.1007/s12035-020-01887-5. Epub 2020 Feb 15. Mol Neurobiol. 2020. PMID: 32062842
-
Cyclin C/cdk3 promotes Rb-dependent G0 exit.Cell. 2004 Apr 16;117(2):239-51. doi: 10.1016/s0092-8674(04)00300-9. Cell. 2004. PMID: 15084261
-
Neuronal apoptosis at the G1/S cell cycle checkpoint.Cell Tissue Res. 2001 Aug;305(2):217-28. doi: 10.1007/s004410100396. Cell Tissue Res. 2001. PMID: 11545259 Review.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
Cited by
-
Cyclin C stimulates β-cell proliferation in rat and human pancreatic β-cells.Am J Physiol Endocrinol Metab. 2015 Mar 15;308(6):E450-9. doi: 10.1152/ajpendo.00260.2014. Epub 2015 Jan 6. Am J Physiol Endocrinol Metab. 2015. PMID: 25564474 Free PMC article.
-
Failure of DNA double-strand break repair by tau mediates Alzheimer's disease pathology in vitro.Commun Biol. 2022 Apr 13;5(1):358. doi: 10.1038/s42003-022-03312-0. Commun Biol. 2022. PMID: 35418705 Free PMC article.
-
Cyclin-dependent kinase inhibitors in brain cancer: current state and future directions.Cancer Drug Resist. 2020 Mar 19;3(1):48-62. doi: 10.20517/cdr.2019.105. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582046 Free PMC article. Review.
-
Senescent brain cell types in Alzheimer's disease: Pathological mechanisms and therapeutic opportunities.Neurotherapeutics. 2025 Apr;22(3):e00519. doi: 10.1016/j.neurot.2024.e00519. Epub 2025 Jan 6. Neurotherapeutics. 2025. PMID: 39765417 Free PMC article. Review.
-
Physiological and pathophysiological functions of cell cycle proteins in post-mitotic neurons: implications for Alzheimer's disease.Acta Neuropathol. 2015 Apr;129(4):511-25. doi: 10.1007/s00401-015-1382-7. Epub 2015 Jan 25. Acta Neuropathol. 2015. PMID: 25618528 Free PMC article. Review.
References
-
- Bassing CH, Alt FW. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst) 2004;3:781–796. - PubMed
-
- Lavin MF, Kozlov S. DNA damage-induced signalling in ataxia-telangiectasia and related syndromes. Radiother Oncol. 2007;83:231–237. - PubMed
-
- Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutat Res. 2007;614:24–36. - PubMed
-
- Biton S, Barzilai A, Shiloh Y. The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA Repair (Amst) 2008;7:1028–1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

