Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B' HIV-1 candidate vaccine
- PMID: 20111582
- PMCID: PMC2799527
- DOI: 10.1371/journal.pone.0008617
Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B' HIV-1 candidate vaccine
Abstract
Background: We conducted a Phase I dose escalation trial of ADVAX, a DNA-based candidate HIV-1 vaccine expressing Clade C/B' env, gag, pol, nef, and tat genes. Sequences were derived from a prevalent circulating recombinant form in Yunnan, China, an area of high HIV-1 incidence. The objective was to evaluate the safety and immunogenicity of ADVAX in human volunteers.
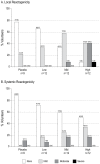
Methodology/principal findings: ADVAX or placebo was administered intramuscularly at months 0, 1 and 3 to 45 healthy volunteers not at high risk for HIV-1. Three dosage levels [0.2 mg (low), 1.0 mg (mid), and 4.0 mg (high)] were tested. Twelve volunteers in each dosage group were assigned to receive ADVAX and three to receive placebo in a double-blind design. Subjects were followed for local and systemic reactogenicity, adverse events, and clinical laboratory parameters. Study follow up was 18 months. Humoral immunogenicity was evaluated by anti-gp120 binding ELISA. Cellular immunogenicity was assessed by a validated IFNgamma ELISpot assay and intracellular cytokine staining. ADVAX was safe and well-tolerated, with no vaccine-related serious adverse events. Local and systemic reactogenicity events were reported by 64% and 42% of vaccine recipients, respectively. The majority of events were mild. The IFNgamma ELISpot response rates to any HIV antigen were 0/9 (0%) in the placebo group, 3/12 (25%) in the low-dosage group, 4/12 (33%) in the mid-dosage group, and 2/12 (17%) in the high-dosage group. Overall, responses were generally transient and occurred to each gene product, although volunteers responded to single antigens only. Binding antibodies to gp120 were not detected in any volunteers, and HIV seroconversion did not occur.
Conclusions/significance: ADVAX delivered intramuscularly is safe, well-tolerated, and elicits modest but transient cellular immune responses.
Trial registration: Clinicaltrials.gov NCT00249106.
Conflict of interest statement
Figures
Similar articles
-
Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B'/C candidate vaccine.PLoS One. 2010 Jan 25;5(1):e8816. doi: 10.1371/journal.pone.0008816. PLoS One. 2010. PMID: 20111599 Free PMC article. Clinical Trial.
-
Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate.PLoS One. 2010 Nov 15;5(11):e13983. doi: 10.1371/journal.pone.0013983. PLoS One. 2010. PMID: 21085591 Free PMC article. Clinical Trial.
-
A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults.PLoS One. 2012;7(8):e41936. doi: 10.1371/journal.pone.0041936. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22870265 Free PMC article. Clinical Trial.
-
A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers.AIDS Res Hum Retroviruses. 2009 Nov;25(11):1107-16. doi: 10.1089/aid.2009.0096. AIDS Res Hum Retroviruses. 2009. PMID: 19943789 Clinical Trial.
-
Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa.PLoS One. 2010 Sep 21;5(9):e12873. doi: 10.1371/journal.pone.0012873. PLoS One. 2010. PMID: 20877623 Free PMC article. Clinical Trial.
Cited by
-
Novel directions in HIV-1 vaccines revealed from clinical trials.Curr Opin HIV AIDS. 2013 Sep;8(5):421-31. doi: 10.1097/COH.0b013e3283632c26. Curr Opin HIV AIDS. 2013. PMID: 23743791 Free PMC article. Review.
-
Elevated Numbers of HIV-Specific Poly-Functional CD8+ T Cells With Stem Cell-Like and Follicular Homing Phenotypes in HIV-Exposed Seronegative Individuals.Front Immunol. 2021 Mar 15;12:638144. doi: 10.3389/fimmu.2021.638144. eCollection 2021. Front Immunol. 2021. PMID: 33889151 Free PMC article.
-
Induction and maintenance of bi-functional (IFN-γ + IL-2+ and IL-2+ TNF-α+) T cell responses by DNA prime MVA boosted subtype C prophylactic vaccine tested in a Phase I trial in India.PLoS One. 2019 Mar 28;14(3):e0213911. doi: 10.1371/journal.pone.0213911. eCollection 2019. PLoS One. 2019. PMID: 30921340 Free PMC article. Clinical Trial.
-
Nucleic acid-based vaccine platforms against the coronavirus disease 19 (COVID-19).Arch Microbiol. 2023 Mar 30;205(4):150. doi: 10.1007/s00203-023-03480-5. Arch Microbiol. 2023. PMID: 36995507 Free PMC article. Review.
-
Recombinant protein vaccines, a proven approach against coronavirus pandemics.Adv Drug Deliv Rev. 2021 Mar;170:71-82. doi: 10.1016/j.addr.2021.01.001. Epub 2021 Jan 7. Adv Drug Deliv Rev. 2021. PMID: 33421475 Free PMC article. Review.
References
-
- UNAIDS. AIDS Epidemic Update: December 2007. UNAIDS/07.27E/JC1322E.
-
- Wei L, Chen J, Rodolph M, Beauchamp G, Masse B, et al. HIV incidence, retention, and changes of high-risk behaviors among rural injection drug users in Guangxi, China. Subst Abus. 2006;27:53–61. - PubMed
-
- Huang Y, Chen Z, Zhang W, Gurner D, Song Y, et al. Design, construction, and characterization of a dual-promoter multigenic DNA vaccine directed against an HIV-1 subtype C/B' recombinant. J Acquir Immune Defic Syndr. 2008;47:403–411. - PubMed

