Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B'/C candidate vaccine
- PMID: 20111599
- PMCID: PMC2810329
- DOI: 10.1371/journal.pone.0008816
Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B'/C candidate vaccine
Abstract
Background: We conducted a Phase I dose-escalation trial of ADMVA, a Clade-B'/C-based HIV-1 candidate vaccine expressing env, gag, pol, nef, and tat in a modified vaccinia Ankara viral vector. Sequences were derived from a prevalent circulating HIV-1 recombinant form in Yunnan, China, an area of high HIV incidence. The objective was to evaluate the safety and immunogenicity of ADMVA in human volunteers.
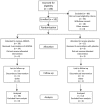
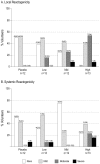
Methodology/principal findings: ADMVA or placebo was administered intramuscularly at months 0, 1 and 6 to 50 healthy adult volunteers not at high risk for HIV-1. In each dosage group [1x10(7) (low), 5x10(7) (mid), or 2.5x10(8) pfu (high)] volunteers were randomized in a 3:1 ratio to receive ADMVA or placebo in a double-blinded design. Subjects were followed for local and systemic reactogenicity, adverse events including cardiac adverse events, and clinical laboratory parameters. Study follow up was 18 months. Humoral immunogenicity was evaluated by anti-gp120 binding ELISA, immunoflourescent staining, and HIV-1 neutralization. Cellular immunogenicity was assessed by a validated IFNgamma ELISpot assay and intracellular cytokine staining. Anti-vaccinia binding titers were measured by ELISA. ADMVA was generally well-tolerated, with no vaccine-related serious adverse events or cardiac adverse events. Local or systemic reactogenicity events were reported by 77% and 78% of volunteers, respectively. The majority of events were of mild intensity. The IFNgamma ELISpot response rate to any HIV antigen was 0/12 (0%) in the placebo group, 3/12 (25%) in the low dosage group, 6/12 (50%) in the mid dosage group, and 8/13 (62%) in the high dosage group. Responses were often multigenic and occasionally persisted up to one year post vaccination. Antibodies to gp120 were detected in 0/12 (0%), 8/13 (62%), 6/12 (50%) and 10/13 (77%) in the placebo, low, mid, and high dosage groups, respectively. Antibodies persisted up to 12 months after vaccination, with a trend toward agreement with the ability to neutralize HIV-1 SF162 in vitro. Two volunteers mounted antibodies that were able to neutralize clade-matched viruses.
Conclusions/significance: ADMVA was well-tolerated and elicited durable humoral and cellular immune responses.
Trial registration: Clinicaltrials.gov NCT00252148.
Conflict of interest statement
Figures
Similar articles
-
Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B' HIV-1 candidate vaccine.PLoS One. 2010 Jan 25;5(1):e8617. doi: 10.1371/journal.pone.0008617. PLoS One. 2010. PMID: 20111582 Free PMC article. Clinical Trial.
-
Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate.PLoS One. 2010 Nov 15;5(11):e13983. doi: 10.1371/journal.pone.0013983. PLoS One. 2010. PMID: 21085591 Free PMC article. Clinical Trial.
-
A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers.AIDS Res Hum Retroviruses. 2009 Nov;25(11):1107-16. doi: 10.1089/aid.2009.0096. AIDS Res Hum Retroviruses. 2009. PMID: 19943789 Clinical Trial.
-
Design, construction, and characterization of a multigenic modified vaccinia Ankara candidate vaccine against human immunodeficiency virus type 1 subtype C/B'.J Acquir Immune Defic Syndr. 2008 Apr 1;47(4):412-21. doi: 10.1097/QAI.0b013e3181651bb2. J Acquir Immune Defic Syndr. 2008. PMID: 18209682
-
Safety and immunogenicity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 subtype B (MVA-B) in healthy HIV-1-uninfected volunteers: A phase I clinical trial (RISVAC02).Vaccine. 2011 Oct 26;29(46):8309-16. doi: 10.1016/j.vaccine.2011.08.098. Epub 2011 Sep 9. Vaccine. 2011. PMID: 21907749 Clinical Trial.
Cited by
-
Enhancing vaccine antibody responses by targeting Clec9A on dendritic cells.NPJ Vaccines. 2017 Nov 6;2:31. doi: 10.1038/s41541-017-0033-5. eCollection 2017. NPJ Vaccines. 2017. PMID: 29263886 Free PMC article.
-
Prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia Ankara vaccines: a systematic review.PLoS One. 2013;8(1):e54407. doi: 10.1371/journal.pone.0054407. Epub 2013 Jan 17. PLoS One. 2013. PMID: 23349878 Free PMC article.
-
DNA Vaccination by Electroporation Amplifies Broadly Cross-Restricted Public TCR Clonotypes Shared with HIV Controllers.J Immunol. 2017 Nov 15;199(10):3437-3452. doi: 10.4049/jimmunol.1700953. Epub 2017 Oct 9. J Immunol. 2017. PMID: 28993513 Free PMC article.
-
Deletion of the vaccinia virus N2L gene encoding an inhibitor of IRF3 improves the immunogenicity of modified vaccinia virus Ankara expressing HIV-1 antigens.J Virol. 2014 Mar;88(6):3392-410. doi: 10.1128/JVI.02723-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390336 Free PMC article.
-
Vaccination with Combination DNA and Virus-Like Particles Enhances Humoral and Cellular Immune Responses upon Boost with Recombinant Modified Vaccinia Virus Ankara Expressing Human Immunodeficiency Virus Envelope Proteins.Vaccines (Basel). 2017 Dec 19;5(4):52. doi: 10.3390/vaccines5040052. Vaccines (Basel). 2017. PMID: 29257056 Free PMC article.
References
-
- UNAIDS (2007) AIDS Epidemic Update: December 2007. UNAIDS/07.27E/ JC1322E.
-
- Wei L, Chen J, Rodolph M, Beauchamp G, Masse B, et al. HIV incidence, retention, and changes of high-risk behaviors among rural injection drug users in Guangxi, China. Subst Abus. 2006;27:53–61. - PubMed
-
- Huang Y, Chen Z, Zhang W, Gurner D, Song Y, et al. Design, construction, and characterization of a dual-promoter multigenic DNA vaccine directed against an HIV-1 subtype C/B' recombinant. J Acquir Immune Defic Syndr. 2008;47:403–411. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

