Effect of Potato virus Y on the NADP-malic enzyme from Nicotiana tabacum L.: mRNA, expressed protein and activity
- PMID: 20111689
- PMCID: PMC2812832
- DOI: 10.3390/ijms10083583
Effect of Potato virus Y on the NADP-malic enzyme from Nicotiana tabacum L.: mRNA, expressed protein and activity
Abstract
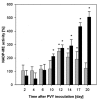
The effect of biotic stress induced by viral infection (Potato virus Y, strain NTN and O) on NADP-malic enzyme (EC 1.1.1.40) in tobacco plants (Nicotiana tabacum L., cv. Petit Havana, SR1) was tested at the transcriptional, translational and activity level. The increase of enzyme activity in infected leaves was correlated with the increased amount of expressed protein and with mRNA of cytosolic NADP-ME isoform. Transcription of the chloroplastic enzyme was not influenced by viral infection. The increase of the enzyme activity was also detected in stems and roots of infected plants. The effect of viral infection induced by Potato virus Y, NTN strain, causing more severe symptoms, was compared with the effect induced by milder strain PVY(O). The observed increase in NADP-malic enzyme activity in all parts of the studied plants was higher in the case of PVY(NTN) strain than in the case of strain PVY(O). The relevance of NADP-malic enzyme in plants under stress conditions was discussed.
Keywords: NADP-malic enzyme; Nicotiana tabacum L.; Potato virus Y strain NTN (PVYNTN); Potato virus Y strain O (PVYO); biotic stress; real time-PCR.
Figures



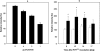
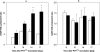

Similar articles
-
The activity and isoforms of NADP-malic enzyme in Nicotiana benthamiana plants under biotic stress.Gen Physiol Biophys. 2007 Dec;26(4):281-9. Gen Physiol Biophys. 2007. PMID: 18281746
-
Phosphoenolpyruvate carboxylase, NADP-malic enzyme, and pyruvate, phosphate dikinase are involved in the acclimation of Nicotiana tabacum L. to drought stress.J Plant Physiol. 2014 Mar 1;171(5):19-25. doi: 10.1016/j.jplph.2013.10.017. Epub 2013 Dec 11. J Plant Physiol. 2014. PMID: 24484954
-
Host recovery and reduced virus level in the upper leaves after Potato virus Y infection occur in tobacco and tomato but not in potato plants.Viruses. 2015 Feb 11;7(2):680-98. doi: 10.3390/v7020680. Viruses. 2015. PMID: 25679498 Free PMC article.
-
Occurrence of Potato Tuber Necrotic Isolates of Potato virus Y in a Commercial Tobacco Field in Southern Ontario, Canada.Plant Dis. 2008 Nov;92(11):1586. doi: 10.1094/PDIS-92-11-1586B. Plant Dis. 2008. PMID: 30764466
-
Roles of malic enzymes in plant development and stress responses.Plant Signal Behav. 2019;14(10):e1644596. doi: 10.1080/15592324.2019.1644596. Epub 2019 Jul 19. Plant Signal Behav. 2019. PMID: 31322479 Free PMC article. Review.
Cited by
-
Potyviral Helper-Component Protease: Multifaced Functions and Interactions with Host Proteins.Plants (Basel). 2024 Apr 29;13(9):1236. doi: 10.3390/plants13091236. Plants (Basel). 2024. PMID: 38732454 Free PMC article. Review.
-
Partially resistant Cucurbita pepo showed late onset of the Zucchini yellow mosaic virus infection due to rapid activation of defense mechanisms as compared to susceptible cultivar.Front Plant Sci. 2015 Apr 28;6:263. doi: 10.3389/fpls.2015.00263. eCollection 2015. Front Plant Sci. 2015. PMID: 25972878 Free PMC article.
-
Phytoremediation of carbamazepine and its metabolite 10,11-epoxycarbamazepine by C3 and C4 plants.Environ Sci Pollut Res Int. 2015 Dec;22(24):20271-82. doi: 10.1007/s11356-015-5190-3. Epub 2015 Aug 27. Environ Sci Pollut Res Int. 2015. PMID: 26310701
References
-
- Arias MC, Lenardon S, Taleisnik E. Carbon metabolism alterations in sunflower plants infected with the Sunflower chlorotic mottle virus. J. Phytopathol. 2003;151:267–273.
-
- Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. - PubMed
-
- Hammond-Kosack KH, Jones JDG. Responses to plant pathogens. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry & Molecular Biology of Plants. 4th ed. American Society of Plant Physiologists; Rockville, MD, USA: 2002. pp. 1102–1152.
-
- Maurino VG, Saigo M, Andreo CS, Drincovich MF. Non-photosynthetic ‘malic enzyme’ from maize: A constitutively expressed enzyme that responds to plant defence inducers. Plant Mol. Biol. 2001;45:409–420. - PubMed
-
- Synkova H, Valcke R. Response to mild water stress in transgenic Pssu-ipt tobacco. Physiol. Plant. 2001;112:513–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

