Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction
- PMID: 20111696
- PMCID: PMC2811559
- DOI: 10.2119/molmed.2009.00109
Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction
Abstract
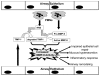
Cystic fibrosis (CF) is a lethal genetic disorder characterized by airway remodeling and inflammation, leading to premature death. Recent evidence suggests the importance of protease activity in CF pathogenesis. One prominent protease, matrix metalloprotease (MMP)-9, demonstrates increased activity in CF individuals undergoing acute pulmonary exacerbation. This is thought to be mediated by both direct MMP-9 activation and the degradation of its natural inhibitor, tissue inhibitor of metalloprotease-1 (TIMP-1). To examine if this relationship exists in nonexacerbating CF individuals, we examined protease activity in sputum from these individuals compared with nondisease controls. We demonstrated increased gelatinolytic activity in CF sputum. These samples had elevated human neutrophil elastase (HNE) levels which correlated with an increased MMP-9/TIMP-1 ratio. To determine if HNE could discretely cleave and activate MMP-9, these enzymes were coincubated and two specific cleavage sites, between Valine(38) and Alanine(39), and between Alanine (39) and glutamic acid(40) were observed. These sites corresponded with appropriate molecular weight for the activated MMP-9 isoform in CF sputum. Using N-terminal sequencing of cleavage fragments obtained with TIMP-1 incubation with HNE, we confirmed the TIMP-1 cleavage site for HNE is at Valine(69)-Cysteine(70). We also show for the first time that human neutrophils were capable of degrading TIMP-1 ex vivo and that a 16 kDa TIMP-1 fragment was identified in CF sputum, consistent with the expected cleavage of TIMP-1 by HNE. These results demonstrate increased MMP-9 activity in stable CF lung disease, and the presence of specific protease products in CF sputum highlights that HNE-mediated activity plays a role in this dysregulation.
Figures

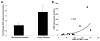

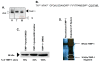

References
-
- Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–58. - PubMed
-
- Gadek JD, Pacht ER. The protease-antiprotease balance within the human lung. Lung. 1990;168(Suppl):552–64. - PubMed
-
- Stoller J, Aboussouan L. Alpha1-antitrypsin deficiency. Lancet. 2005;365:2225–36. - PubMed
-
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

