The coming-out of malaria gametocytes
- PMID: 20111746
- PMCID: PMC2810480
- DOI: 10.1155/2010/976827
The coming-out of malaria gametocytes
Abstract
The tropical disease malaria, which results in more than one million deaths annually, is caused by protozoan parasites of the genus Plasmodium and transmitted by blood-feeding Anopheline mosquitoes. Parasite transition from the human host to the mosquito vector is mediated by gametocytes, sexual stages that are formed in human erythrocytes, which therefore play a crucial part in the spread of the tropical disease. The uptake by the blood-feeding mosquito triggers important molecular and cellular changes in the gametocytes, thus mediating the rapid adjustment of the parasite from the warm-blooded host to the insect host and subsequently initiating reproduction. The contact with midgut factors triggers gametocyte activation and results in their egress from the enveloping erythrocyte, which then leads to gamete formation and fertilization. This review summarizes recent findings on the role of gametocytes during transmission to the mosquito and particularly focuses on the molecular mechanisms underlying gametocyte activation and emergence from the host erythrocyte during gametogenesis.
Figures
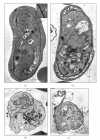
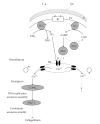
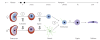
Similar articles
-
Malaria parasite development in mosquitoes.Annu Rev Entomol. 1998;43:519-43. doi: 10.1146/annurev.ento.43.1.519. Annu Rev Entomol. 1998. PMID: 9444756 Review.
-
The development of malaria parasites in the mosquito midgut.Cell Microbiol. 2016 Jul;18(7):905-18. doi: 10.1111/cmi.12604. Epub 2016 May 24. Cell Microbiol. 2016. PMID: 27111866 Free PMC article. Review.
-
Changes in the transcriptome of the malaria parasite Plasmodium falciparum during the initial phase of transmission from the human to the mosquito.BMC Genomics. 2013 Apr 15;14:256. doi: 10.1186/1471-2164-14-256. BMC Genomics. 2013. PMID: 23586929 Free PMC article.
-
The Plasmodium bottleneck: malaria parasite losses in the mosquito vector.Mem Inst Oswaldo Cruz. 2014 Aug;109(5):644-61. doi: 10.1590/0074-0276130597. Mem Inst Oswaldo Cruz. 2014. PMID: 25185005 Free PMC article.
-
Effects of the antimalarial drugs ferroquine and artesunate on Plasmodium yoelii yoelii gametocytegenesis and vectorial transmission.Sante. 2011 Jul-Sep;21(3):133-42. doi: 10.1684/san.2011.0261. Sante. 2011. PMID: 22294247
Cited by
-
Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development.PLoS Pathog. 2012;8(10):e1002964. doi: 10.1371/journal.ppat.1002964. Epub 2012 Oct 18. PLoS Pathog. 2012. PMID: 23093935 Free PMC article.
-
The Plasmodium falciparum CCCH Zinc Finger Protein ZNF4 Plays an Important Role in Gametocyte Exflagellation through the Regulation of Male Enriched Transcripts.Cells. 2022 May 17;11(10):1666. doi: 10.3390/cells11101666. Cells. 2022. PMID: 35626703 Free PMC article.
-
The s48/45 six-cysteine proteins: mediators of interaction throughout the Plasmodium life cycle.Int J Parasitol. 2017 Jun;47(7):409-423. doi: 10.1016/j.ijpara.2016.10.002. Epub 2016 Nov 27. Int J Parasitol. 2017. PMID: 27899328 Free PMC article. Review.
-
Protein phosphorylation during Plasmodium berghei gametogenesis.Exp Parasitol. 2015 Sep;156:49-60. doi: 10.1016/j.exppara.2015.05.010. Epub 2015 May 22. Exp Parasitol. 2015. PMID: 26008612 Free PMC article.
-
Expression of a type B RIFIN in Plasmodium falciparum merozoites and gametes.Malar J. 2012 Dec 21;11:429. doi: 10.1186/1475-2875-11-429. Malar J. 2012. PMID: 23259643 Free PMC article.
References
-
- Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007;134(14):1911–1929. - PubMed
-
- Carlton JM, Angiuoli SV, Suh BB, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002;419(6906):512–519. - PubMed
-
- Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419(6906):520–526. - PubMed
-
- Lasonder E, Ishihama Y, Andersen JS, et al. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spedrometry. Nature. 2002;419(6906):537–542. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

