Deimination is regulated at multiple levels including auto-deimination of peptidylarginine deiminases
- PMID: 20111885
- PMCID: PMC11115946
- DOI: 10.1007/s00018-010-0262-5
Deimination is regulated at multiple levels including auto-deimination of peptidylarginine deiminases
Abstract
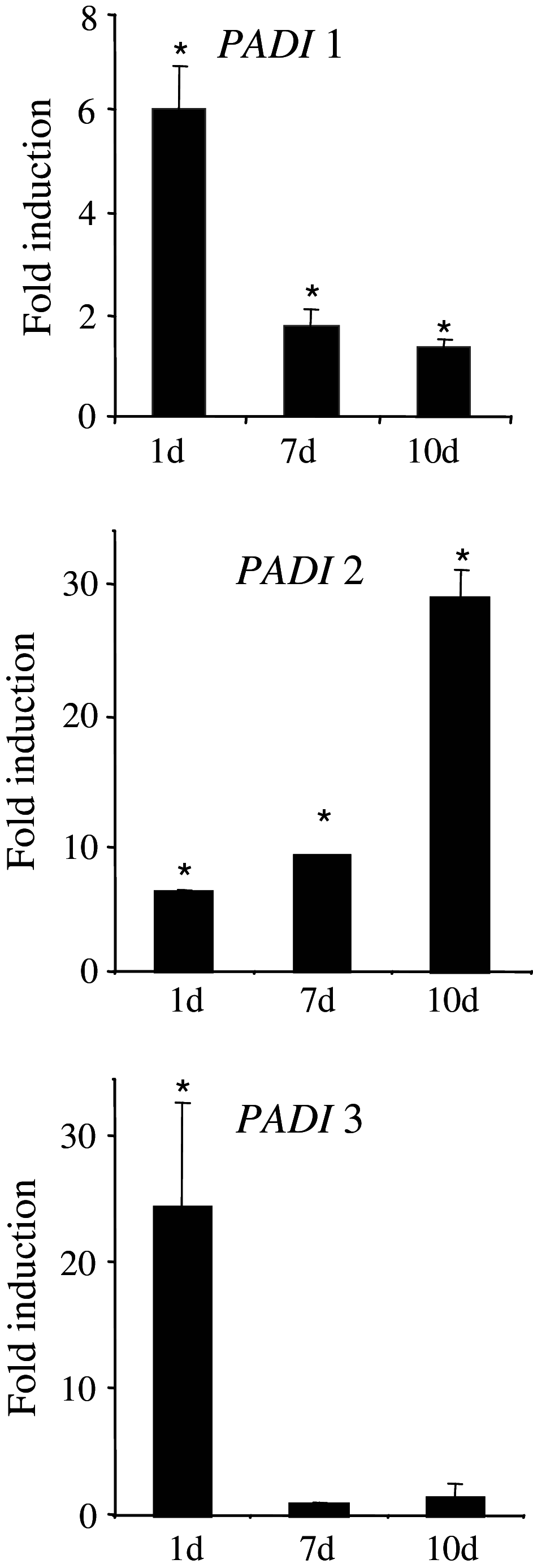
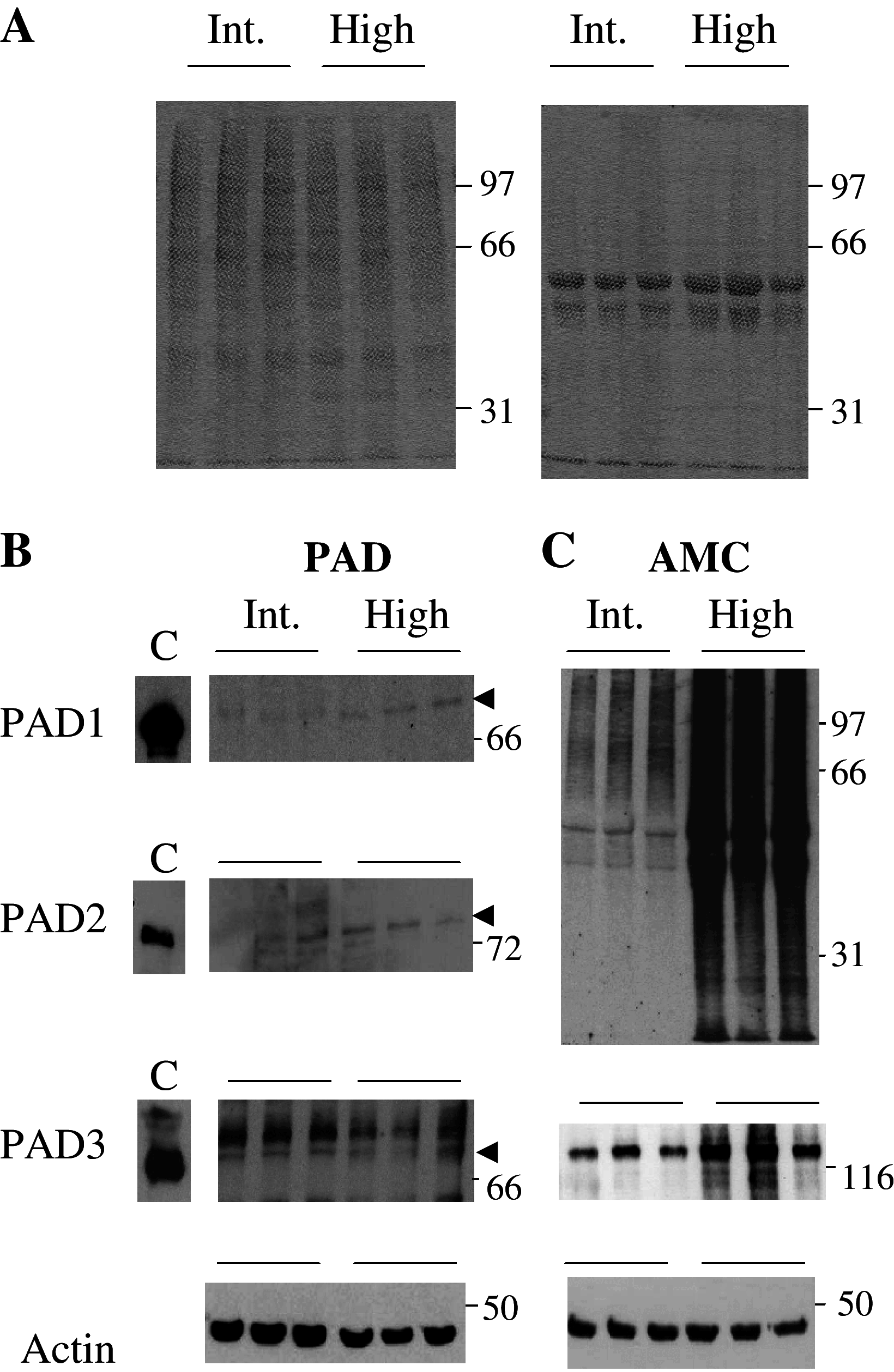
Peptidylarginine deiminases (PADs) catalyze deimination, converting arginyl to citrullyl residues. Only three PAD isotypes are detected in the epidermis where they play a crucial role, targeting filaggrin, a key actor for the tissue hydration and barrier functions. Their expression and activation depends on the keratinocyte differentiation state. To investigate this regulation, we used primary keratinocytes induced to differentiate either by increasing cell-density or by treatment with vitamin D. High cell-density increased PAD1 and 3, but not PAD2, at the mRNA and protein levels, and up-regulated protein deimination. By contrast, vitamin D increased PAD1-3 mRNA amounts, with distinct kinetics, but neither the proteins nor the deimination rate. Furthermore, auto-deimination was shown to decrease PAD activity, increasing the distances between the four major amino acids of the active site. In summary, deimination can be regulated at multiple levels: transcription of the PADI genes, translation of the corresponding mRNAs, and auto-deimination of PADs.
Figures
Similar articles
-
Peptidylarginine deiminase isoforms 1-3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin.J Invest Dermatol. 2005 Feb;124(2):384-93. doi: 10.1111/j.0022-202X.2004.23568.x. J Invest Dermatol. 2005. PMID: 15675958
-
Acefylline activates filaggrin deimination by peptidylarginine deiminases in the upper epidermis.J Dermatol Sci. 2016 Feb;81(2):101-6. doi: 10.1016/j.jdermsci.2015.11.006. Epub 2015 Nov 17. J Dermatol Sci. 2016. PMID: 26616205
-
Lowering relative humidity level increases epidermal protein deimination and drives human filaggrin breakdown.J Dermatol Sci. 2017 May;86(2):106-113. doi: 10.1016/j.jdermsci.2017.02.280. Epub 2017 Feb 20. J Dermatol Sci. 2017. PMID: 28242341 Free PMC article.
-
Peptidylarginine deiminases and deiminated proteins at the epidermal barrier.Exp Dermatol. 2018 Aug;27(8):852-858. doi: 10.1111/exd.13684. Epub 2018 Jun 29. Exp Dermatol. 2018. PMID: 29756256 Review.
-
Deimination in epidermal barrier and hair formation.Philos Trans R Soc Lond B Biol Sci. 2023 Nov 20;378(1890):20220245. doi: 10.1098/rstb.2022.0245. Epub 2023 Oct 2. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37778378 Free PMC article. Review.
Cited by
-
PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets.Nat Rev Rheumatol. 2020 Jun;16(6):301-315. doi: 10.1038/s41584-020-0409-1. Epub 2020 Apr 27. Nat Rev Rheumatol. 2020. PMID: 32341463 Review.
-
Activation of PAD4 in NET formation.Front Immunol. 2012 Nov 29;3:360. doi: 10.3389/fimmu.2012.00360. eCollection 2012. Front Immunol. 2012. PMID: 23264775 Free PMC article.
-
Peptidylarginine Deiminase Inhibitor Cl-Amidine Attenuates Cornification and Interferes with the Regulation of Autophagy in Reconstructed Human Epidermis.J Invest Dermatol. 2019 Sep;139(9):1889-1897.e4. doi: 10.1016/j.jid.2019.02.026. Epub 2019 Mar 13. J Invest Dermatol. 2019. PMID: 30878672 Free PMC article.
-
Redox-Mediated Carbamylation As a Hapten Model Applied to the Origin of Antibodies to Modified Proteins in Rheumatoid Arthritis.Antioxid Redox Signal. 2022 Mar;36(7-9):389-409. doi: 10.1089/ars.2021.0064. Epub 2021 Jun 4. Antioxid Redox Signal. 2022. PMID: 33906423 Free PMC article. Review.
-
VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression.Mol Endocrinol. 2012 Jan;26(1):37-51. doi: 10.1210/me.2011-1109. Epub 2011 Nov 22. Mol Endocrinol. 2012. PMID: 22108803 Free PMC article.
References
-
- Méchin MC, Sebbag M, Arnaud J, Nachat R, Foulquier C, Adoue V, Coudane F, Duplan H, Schmitt AM, Chavanas S, Guerrin M, Serre G, Simon M. Update on peptidylarginine deiminases and deimination in skin physiology and severe human diseases. Int J Cosmet Sci. 2007;29:147–168. doi: 10.1111/j.1467-2494.2007.00377.x. - DOI - PubMed
-
- Sebbag M, Chapuy-Regaud S, Auger I, Petit-Texeira E, Clavel C, Nogueira L, Vincent C, Cornelis F, Roudier J, Serre G. Clinical and pathophysiological significance of the autoimmune response to citrullinated proteins in rheumatoid arthritis. Joint Bone Spine. 2004;71:493–502. doi: 10.1016/j.jbspin.2004.07.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

