p66Shc--a longevity redox protein in human prostate cancer progression and metastasis : p66Shc in cancer progression and metastasis
- PMID: 20111892
- PMCID: PMC2909029
- DOI: 10.1007/s10555-010-9213-8
p66Shc--a longevity redox protein in human prostate cancer progression and metastasis : p66Shc in cancer progression and metastasis
Abstract
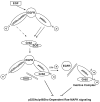
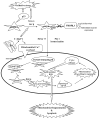
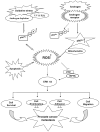
p66Shc, a 66 kDa proto-oncogene Src homologous-collagen homologue (Shc) adaptor protein, is classically known in mediating receptor tyrosine kinase signaling and recently identified as a sensor to oxidative stress-induced apoptosis and as a longevity protein in mammals. The expression of p66Shc is decreased in mice and increased in human fibroblasts upon aging and in aging-related diseases, including prostate cancer. p66Shc protein level correlates with the proliferation of several carcinoma cells and can be regulated by steroid hormones. Recent advances point that p66Shc protein plays a role in mediating cross-talk between steroid hormones and redox signals by serving as a common convergence point in signaling pathways on cell proliferation and apoptosis. This article first reviews the unique function of p66Shc protein in regulating oxidative stress-induced apoptosis. Subsequently, we discuss its novel role in androgen-regulated prostate cancer cell proliferation and metastasis and the mechanism by which it mediates androgen action via the redox signaling pathway. The data together indicate that p66Shc might be a useful biomarker for the prognosis of prostate cancer and serve as an effective target for its cancer treatment.
Figures

Similar articles
-
Reactive oxygen species induced by p66Shc longevity protein mediate nongenomic androgen action via tyrosine phosphorylation signaling to enhance tumorigenicity of prostate cancer cells.Free Radic Biol Med. 2012 Jul 1;53(1):95-108. doi: 10.1016/j.freeradbiomed.2012.03.024. Epub 2012 Apr 16. Free Radic Biol Med. 2012. PMID: 22561705 Free PMC article.
-
Steroids up-regulate p66Shc longevity protein in growth regulation by inhibiting its ubiquitination.PLoS One. 2011 Jan 14;6(1):e15942. doi: 10.1371/journal.pone.0015942. PLoS One. 2011. PMID: 21264241 Free PMC article.
-
Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells.Oncogene. 2008 Aug 28;27(37):5057-68. doi: 10.1038/onc.2008.143. Epub 2008 May 26. Oncogene. 2008. PMID: 18504439 Free PMC article.
-
p66Shc, a multifaceted protein linking Erk signalling, glucose metabolism, and oxidative stress.Arch Physiol Biochem. 2011 Jul;117(3):116-24. doi: 10.3109/13813455.2011.562513. Epub 2011 Apr 21. Arch Physiol Biochem. 2011. PMID: 21506908 Review.
-
The interplay between p66Shc, reactive oxygen species and cancer cell metabolism.Eur J Clin Invest. 2015 Jan;45 Suppl 1:25-31. doi: 10.1111/eci.12364. Eur J Clin Invest. 2015. PMID: 25524583 Review.
Cited by
-
Prognostic value and prospective molecular mechanism of miR-100-5p in hepatocellular carcinoma: A comprehensive study based on 1,258 samples.Oncol Lett. 2019 Dec;18(6):6126-6142. doi: 10.3892/ol.2019.10962. Epub 2019 Oct 4. Oncol Lett. 2019. PMID: 31788087 Free PMC article.
-
Emerging Proteins in CRPC: Functional Roles and Clinical Implications.Front Oncol. 2022 Jun 10;12:873876. doi: 10.3389/fonc.2022.873876. eCollection 2022. Front Oncol. 2022. PMID: 35756667 Free PMC article. Review.
-
Interaction of Mitochondria with the Endoplasmic Reticulum and Plasma Membrane in Calcium Homeostasis, Lipid Trafficking and Mitochondrial Structure.Int J Mol Sci. 2017 Jul 20;18(7):1576. doi: 10.3390/ijms18071576. Int J Mol Sci. 2017. PMID: 28726733 Free PMC article. Review.
-
p66Shc regulates migration of castration-resistant prostate cancer cells.Cell Signal. 2018 Jun;46:1-14. doi: 10.1016/j.cellsig.2018.02.008. Epub 2018 Feb 17. Cell Signal. 2018. PMID: 29462661 Free PMC article.
-
Dynamics of antioxidant heme oxygenase-1 and pro-oxidant p66Shc in promoting advanced prostate cancer progression.Free Radic Biol Med. 2022 Nov 20;193(Pt 1):274-291. doi: 10.1016/j.freeradbiomed.2022.10.269. Epub 2022 Oct 17. Free Radic Biol Med. 2022. PMID: 36265795 Free PMC article.
References
-
- Guo M, Hay BA. Cell proliferation and apoptosis. Current Opinion in Cell Biology. 1999;11:745–752. - PubMed
-
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. - PubMed
-
- Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annual Review of Immunology. 1999;17:781–828. - PubMed
-
- Ginaldi L, De Martinis M, D'Ostilio A, Marini L, Loreto MF, Corsi MP, et al. Cell proliferation and apoptosis in the immune system in the elderly. Immunological Research. 2000;21:31–38. - PubMed
-
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes and Development. 1999;13:1899–1911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

