Genotoxicity of pyrrolizidine alkaloids
- PMID: 20112250
- PMCID: PMC6376482
- DOI: 10.1002/jat.1504
Genotoxicity of pyrrolizidine alkaloids
Abstract
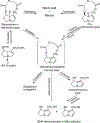
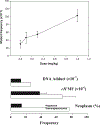
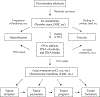
Pyrrolizidine alkaloids (PAs) are common constituents of many plant species around the world. PA-containing plants are probably the most common poisonous plants affecting livestock and wildlife. They can inflict harm to humans through contaminated food sources, herbal medicines and dietary supplements. Half of the identified PAs are genotoxic and many of them are tumorigenic. The mutagenicity of PAs has been extensively studied in different biological systems. Upon metabolic activation, PAs produce DNA adducts, DNA cross-linking, DNA breaks, sister chromatid exchange, micronuclei, chromosomal aberrations, gene mutations and chromosome mutations in vivo and in vitro. PAs induced mutations in the cII gene of rat liver and in the p53 and K-ras genes of mouse liver tumors. It has been suggested that all PAs produce a set of (+/-)-6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine-derived DNA adducts and similar types of gene mutations. The signature types of mutations are G : C --> T : A transversion and tandem base substitutions. Overall, PAs are mutagenic in vivo and in vitro and their mutagenicity appears to be responsible for the carcinogenesis of PAs.
(c) 2010 John Wiley & Sons, Ltd.
Figures


Similar articles
-
Metabolism-mediated cytotoxicity and genotoxicity of pyrrolizidine alkaloids.Arch Toxicol. 2021 Jun;95(6):1917-1942. doi: 10.1007/s00204-021-03060-w. Epub 2021 May 18. Arch Toxicol. 2021. PMID: 34003343 Review.
-
Formation of DHP-derived DNA adducts from metabolic activation of the prototype heliotridine-type pyrrolizidine alkaloid, lasiocarpine.Cancer Lett. 2006 Jan 8;231(1):138-45. doi: 10.1016/j.canlet.2005.01.023. Cancer Lett. 2006. PMID: 16356839
-
1-Formyl-7-hydroxy-6,7-dihydro-5 H-pyrrolizine (1-CHO-DHP): A Potential Proximate Carcinogenic Metabolite of Pyrrolizidine Alkaloids.Chem Res Toxicol. 2019 Jun 17;32(6):1193-1203. doi: 10.1021/acs.chemrestox.9b00038. Epub 2019 Jun 3. Chem Res Toxicol. 2019. PMID: 31120748
-
Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine.Toxicol Lett. 2003 Dec 10;145(3):239-47. doi: 10.1016/s0378-4274(03)00293-5. Toxicol Lett. 2003. PMID: 14580895
-
Hepatotoxicity and tumorigenicity induced by metabolic activation of pyrrolizidine alkaloids in herbs.Curr Drug Metab. 2011 Nov;12(9):823-34. doi: 10.2174/138920011797470119. Curr Drug Metab. 2011. PMID: 21619520 Review.
Cited by
-
Genotoxicity of pyrrolizidine alkaloids in metabolically inactive human cervical cancer HeLa cells co-cultured with human hepatoma HepG2 cells.Arch Toxicol. 2023 Jan;97(1):295-306. doi: 10.1007/s00204-022-03394-z. Epub 2022 Oct 23. Arch Toxicol. 2023. PMID: 36273350 Free PMC article.
-
OCT1-dependent uptake of structurally diverse pyrrolizidine alkaloids in human liver cells is crucial for their genotoxic and cytotoxic effects.Arch Toxicol. 2023 Dec;97(12):3259-3271. doi: 10.1007/s00204-023-03591-4. Epub 2023 Sep 7. Arch Toxicol. 2023. PMID: 37676300 Free PMC article.
-
Cytotoxicity of Eupatorium cannabinum L. ethanolic extract against colon cancer cells and interactions with Bisphenol A and Doxorubicin.BMC Complement Altern Med. 2014 Jul 24;14:264. doi: 10.1186/1472-6882-14-264. BMC Complement Altern Med. 2014. PMID: 25056133 Free PMC article.
-
Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants.Molecules. 2019 Jan 30;24(3):498. doi: 10.3390/molecules24030498. Molecules. 2019. PMID: 30704105 Free PMC article. Review.
-
Structure-Dependent Toxicokinetics of Selected Pyrrolizidine Alkaloids In Vitro.Int J Mol Sci. 2022 Aug 16;23(16):9214. doi: 10.3390/ijms23169214. Int J Mol Sci. 2022. PMID: 36012484 Free PMC article.
References
-
- Allen JR, Hsu IC, Carstens LA. 1975. Dehydroretronecine-induced rhabdomyosarcomas in rats. Cancer Res. 35: 997–1002. - PubMed
-
- Arseculeratne SN, Gunatilaka AA, Panabokke RG. 1981. Studies on medicinal plants of Sri Lanka: occurrence of pyrrolizidine alkaloids and hepatotoxic properties in some traditional medicinal herbs. J. Ethnopharmacol 4: 159–177. - PubMed
-
- Arseculeratne SN, Gunatilaka AA, Panabokke RG. 1985. Studies of medicinal plants of Sri Lanka. Part 14: toxicity of some traditional medicinal herbs. J. Ethnopharmacol 13: 323–335. - PubMed
-
- Arzt J, Mount ME. 1999. Hepatotoxicity associated with pyrrolizidine alkaloid (Crotalaria spp) ingestion in a horse on Easter Island. Vet. Hum. Toxicol 41: 96–99. - PubMed
-
- Bach N, Thung SN, Schaff ner F. 1989. Comfrey herb tea-induced hepatic veno-occlusive disease. Am. J. Med 87: 97–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

