On the general mechanism of selective induction of cytochrome P450 enzymes by chemicals: some theoretical considerations
- PMID: 20113197
- PMCID: PMC2842473
- DOI: 10.1517/17425250903578642
On the general mechanism of selective induction of cytochrome P450 enzymes by chemicals: some theoretical considerations
Abstract
Importance of the field: The CYP isoforms that are selectively induced following exposure to structurally-diverse chemicals often are the ones capable of metabolizing these chemicals. However, the molecular mechanism underlying this apparent functional coupling is not understood at present.
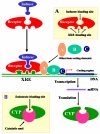
Areas covered in this review: Three hypotheses are developed to explain the complex process of selective chemical induction of CYPs: i) each inducible CYP may have a corresponding intracellular receptor that interacts with the inducer chemical and mediates the selective induction of this CYP; ii) each inducible CYP and its corresponding receptor may share highly similar steric structures for their substrate/inducer-binding sites and iii) each chemically-inducible CYP gene may have distinct genomic response element(s) that interact selectively with the corresponding receptor.
What the reader will gain: The readers are introduced to a novel theoretical framework that offers a plausible mechanistic explanation at the molecular level concerning the complex process of how an organism selectively activates the biosynthesis of certain CYP isoform(s) that can effectively metabolize a chemical to which the organism is exposed.
Take home message: The theoretical framework developed herein seeks to ignite additional critical thinking on this important research subject as well as to promote experimental testing of the proposed theories in the future. Undoubtedly, these studies will enhance the understanding of the molecular mechanisms for the selective induction of CYP enzymes by chemicals.
Figures
Similar articles
-
Induction of cytochromes P450.Curr Top Med Chem. 2004;4(16):1745-66. doi: 10.2174/1568026043387115. Curr Top Med Chem. 2004. PMID: 15579106 Review.
-
P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR.Arch Biochem Biophys. 1999 Sep 1;369(1):11-23. doi: 10.1006/abbi.1999.1351. Arch Biochem Biophys. 1999. PMID: 10462436 Review.
-
Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR).Toxicology. 2009 Feb 4;256(1-2):53-64. doi: 10.1016/j.tox.2008.11.002. Epub 2008 Nov 11. Toxicology. 2009. PMID: 19041682 Free PMC article.
-
Candidate Proficiency Test Chemicals to Address Industrial Chemical Applicability Domains for in vitro Human Cytochrome P450 Enzyme Induction.Front Toxicol. 2022 Jun 20;4:880818. doi: 10.3389/ftox.2022.880818. eCollection 2022. Front Toxicol. 2022. PMID: 35795225 Free PMC article. Review.
-
Gene expression profiles of drug-metabolizing enzymes and transporters with an overexpression of hepatocyte growth factor.Liver Int. 2007 Feb;27(1):109-19. doi: 10.1111/j.1478-3231.2006.01384.x. Liver Int. 2007. PMID: 17241389
Cited by
-
AhR activation underlies the CYP1A autoinduction by A-998679 in rats.Front Genet. 2012 Oct 26;3:213. doi: 10.3389/fgene.2012.00213. eCollection 2012. Front Genet. 2012. PMID: 23112805 Free PMC article.
-
The Multifarious Link between Cytochrome P450s and Cancer.Oxid Med Cell Longev. 2020 Jan 3;2020:3028387. doi: 10.1155/2020/3028387. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 31998435 Free PMC article. Review.
-
Simulation of drug-drug interactions between breast cancer chemotherapeutic agents and antiemetic drugs.Daru. 2023 Dec;31(2):95-105. doi: 10.1007/s40199-023-00463-1. Epub 2023 May 24. Daru. 2023. PMID: 37223851 Free PMC article.
-
Impact of aminophylline on the pharmacodynamics of propofol in beagle dogs.J Pharmacokinet Pharmacodyn. 2014 Dec;41(6):599-612. doi: 10.1007/s10928-014-9377-x. Epub 2014 Aug 24. J Pharmacokinet Pharmacodyn. 2014. PMID: 25150710
-
Toward modular biological models: defining analog modules based on referent physiological mechanisms.BMC Syst Biol. 2014 Aug 16;8:95. doi: 10.1186/s12918-014-0095-1. BMC Syst Biol. 2014. PMID: 25123169 Free PMC article.
References
-
- Gonzalez FJ. Molecular genetics of the P-450 superfamily. Pharmacol Ther. 1990;45:1–38. - PubMed
-
- Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW. P450 superfamily: update on new sequences, gene mapping, accession numbers, and nomenclature. Pharmacogenetics. 1996;6:1–43. - PubMed
-
- Esterbrook R. An introduction to the cytochrome P450s. Mol Aspects Med. 1999;20:5–137. - PubMed
-
- Coon MJ. Cytochrome P450: Nature’s most versatile biological catalyst. Ann Rev Pharmacol Toxicol. 2005;45:1–25. - PubMed
-
- de Montellano PR Ortiz., editor. Cytochrome P-450: Structure, metabolism and biochemistry. Plenum Press; NY: 1986. pp. 1–556.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
