A confocal and electron microscopic comparison of interferon beta-induced changes in vesicular stomatitis virus infection of neuroblastoma and nonneuronal cells
- PMID: 20113203
- PMCID: PMC2833218
- DOI: 10.1089/dna.2009.0963
A confocal and electron microscopic comparison of interferon beta-induced changes in vesicular stomatitis virus infection of neuroblastoma and nonneuronal cells
Abstract
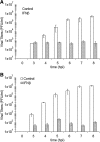
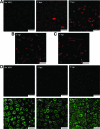
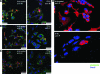
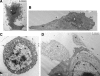
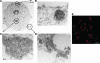
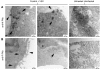
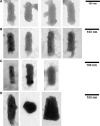
Vesicular stomatitis virus (VSV) replication is highly sensitive to interferon (IFN)-induced antiviral responses. Pretreatment of sensitive cultured cells with IFNbeta results in a 10(4)-fold reduction in the release of infectious VSV particles. However, differences exist between the mechanisms of reduced infectious particle titers in cell lines of neuroblastoma and nonneuronal lineage. In L929-fibroblast-derived cells, using immunofluorescence confocal microscopy, infection under control conditions reveals the accumulation of VSV matrix, phosphoprotein (P), and nucleocapsid (N) proteins over time, with induced cellular morphological changes indicative of cytopathic effects (CPEs). Upon observing L929 cells that had been pretreated with IFNbeta, neither detectable VSV proteins nor CPEs were seen, consistent with type I IFN antiviral protection. When using the same techniques to observe VSV infections of NB41A3 cells, a neuroblastoma cell line, aside from similar viral progression in the untreated control cells, IFNbeta-treated cells illustrated a severely attenuated VSV infection. Attenuated VSV progression was observed through detection of VSV matrix, P, and N proteins in isolated cells during the first 8 h of infection. However, by 18-24 h postinfection all neuroblastomas had succumbed to the viral infection. Finally, upon closer inspection of IFNbeta-treated NB41A3 cells, no detectable changes in VSV protein localization were identified compared with untreated, virally infected neuroblastomas. Next, to extend our study to test our hypothesis that virion assembly is compromised within type I IFN-treated neuroblastoma cells, we employed electron microscopy to examine our experimental conditions at the ultrastructural level. Using VSV-specific antibodies in conjunction with immuno-gold reagents, we observed several similarities between the two cell lines, such as identification of viroplasmic regions containing VSV N and P proteins and signs of stress-induced CPEs of VSV-infected cells, which had either been mock-treated or pretreated with interferon-beta (IFNbeta). One difference we observed between nonneuronal and neuroblastoma cells was more numerous actively budding VSV virions across untreated L929 plasma membranes compared with untreated NB41A3 cells. Additionally, IFNbeta-treated, VSV-infected L929 cells exhibited neither cytoplasmic viroplasm nor viral protein expression. In contrast, IFNbeta-treated, VSV-infected NB41A3 cells showed evidence of VSV infection at a very low frequency as well as small-scale viroplasmic regions that colocalized with viral N and P proteins. Finally, we observed that VSV viral particles harvested from untreated VSV-infected L929 and NB41A3 cells were statistically similar in size and shape. A portion of VSV virions from IFNbeta-treated, virally infected NB41A3 cells were similar in size and shape to virus from both untreated cell types. However, among the sampling of virions, pleomorphic viral particles that were identified from IFNbeta-treated, VSV-infected NB41A3 cells were different enough to suggest a misassembly mechanism as part of the IFNbeta antiviral state in neuroblastoma cells.
Figures





Similar articles
-
IFN-beta-induced alteration of VSV protein phosphorylation in neuronal cells.Viral Immunol. 2009 Dec;22(6):353-69. doi: 10.1089/vim.2009.0057. Viral Immunol. 2009. PMID: 19951173 Free PMC article.
-
VSV replication in neurons is inhibited by type I IFN at multiple stages of infection.Virology. 2005 Mar 15;333(2):215-25. doi: 10.1016/j.virol.2005.01.009. Virology. 2005. PMID: 15721356
-
Radiation Attenuates Prostate Tumor Antiviral Responses to Vesicular Stomatitis Virus Containing IFNβ, Resulting in Pronounced Antitumor Systemic Immune Responses.Mol Cancer Res. 2020 Aug;18(8):1232-1243. doi: 10.1158/1541-7786.MCR-19-0836. Epub 2020 May 4. Mol Cancer Res. 2020. PMID: 32366674
-
Preclinical efficacy of oncolytic VSV-IFNβ in treating cancer: A systematic review.Front Immunol. 2023 Mar 31;14:1085940. doi: 10.3389/fimmu.2023.1085940. eCollection 2023. Front Immunol. 2023. PMID: 37063914 Free PMC article.
-
Induction and function of IFNβ during viral and bacterial infection.Crit Rev Immunol. 2011;31(6):459-74. doi: 10.1615/critrevimmunol.v31.i6.20. Crit Rev Immunol. 2011. PMID: 22321107 Free PMC article. Review.
Cited by
-
Interferon-induced tetherin restricts vesicular stomatitis virus release in neurons.DNA Cell Biol. 2011 Dec;30(12):965-74. doi: 10.1089/dna.2011.1384. Epub 2011 Sep 15. DNA Cell Biol. 2011. PMID: 21919738 Free PMC article.
-
Ultrastructural changes of airway in murine models of allergy and diet-induced metabolic syndrome.ISRN Allergy. 2013 Sep 10;2013:261297. doi: 10.1155/2013/261297. eCollection 2013. ISRN Allergy. 2013. PMID: 24106613 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

