Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever
- PMID: 20113369
- PMCID: PMC2878465
- DOI: 10.1111/j.1365-2567.2009.03224.x
Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever
Abstract
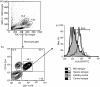
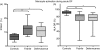
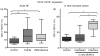
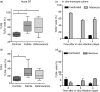
Dengue fever (DF), a public health problem in tropical countries, may present severe clinical manifestations as result of increased vascular permeability and coagulation disorders. Dengue virus (DENV), detected in peripheral monocytes during acute disease and in in vitro infection, leads to cytokine production, indicating that virus-target cell interactions are relevant to pathogenesis. Here we investigated the in vitro and in vivo activation of human peripheral monocytes after DENV infection. The numbers of CD14(+) monocytes expressing the adhesion molecule intercellular adhesion molecule 1 (ICAM-1) were significantly increased during acute DF. A reduced number of CD14(+) human leucocyte antigen (HLA)-DR(+) monocytes was observed in patients with severe dengue when compared to those with mild dengue and controls; CD14(+) monocytes expressing toll-like receptor (TLR)2 and TLR4 were increased in peripheral blood from dengue patients with mild disease, but in vitro DENV-2 infection up-regulated only TLR2. Increased numbers of CD14(+) CD16(+) activated monocytes were found after in vitro and in vivo DENV-2 infection. The CD14(high) CD16(+) monocyte subset was significantly expanded in mild dengue, but not in severe dengue. Increased plasma levels of tumour necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma) and interleukin (IL)-18 in dengue patients were inversely associated with CD14(high) CD16(+), indicating that these cells might be involved in controlling exacerbated inflammatory responses, probably by IL-10 production. We showed here, for the first time, phenotypic changes on peripheral monocytes that were characteristic of cell activation. A sequential monocyte-activation model is proposed in which DENV infection triggers TLR2/4 expression and inflammatory cytokine production, leading eventually to haemorrhagic manifestations, thrombocytopenia, coagulation disorders, plasmatic leakage and shock development, but may also produce factors that act in order to control both intense immunoactivation and virus replication.
Figures



References
-
- Nogueira RMR, de Araújo JMG, Schatzmayr HG. Dengue viruses in Brazil, 1986–2006. Revista Panamericana de Salud Pública/Pan. Am J Public Health. 2007;22:358–63. - PubMed
-
- Brazilian-Health-Ministry Secretaria de Vigilância em Saúde. Boletim epidemiológico, Secretaria de Vigilância em Saúde. Informe Epidemiológico da Dengue. Janeiro a Novembro de 2008. 2009. http://portal.saude.gov.br/portal/arquivos/pdf/boletim_dengue_janeiro_no... [accessed on 26 December 2009]
-
- Avila-Aguero ML, Avila-Aguero CR, Um SL, Soriano-Fallas A, Canas-Coto A, Yan SB. Systemic host inflammatory and coagulation response in the dengue virus primo-infection. Cytokine. 2004;27:173–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

