Effects of nilotinib on regulatory T cells: the dose matters
- PMID: 20113470
- PMCID: PMC2835656
- DOI: 10.1186/1476-4598-9-22
Effects of nilotinib on regulatory T cells: the dose matters
Abstract
Background: Nilotinib is a tyrosine kinase inhibitor with high target specificity. Here, we characterized the effects of nilotinib for the first time on CD4+CD25+ regulatory T cells (Tregs) which regulate anti-tumor/leukemia immune responses.
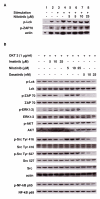
Design and methods: Carboxyfluorescein diacetate succinimidyl ester (CFSE) and 5-bromo-2-deoxy -uridine (BrdU) were used to assess the proliferation and cell cycle distribution of Tregs. The expression of the transcription factor forkhead box P3 (FoxP3) and the glucocorticoid-induced tumor necrosis factor receptor (GITR) were measured by flow cytometry. Western blotting analysis was used to detect the effects of nilotinib on the signal transduction cascade of T-cell receptor (TCR) in Tregs.
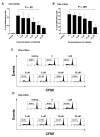
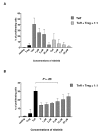
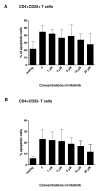
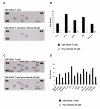
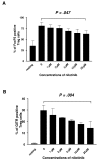
Results: Nilotinib inhibited the proliferation and suppressive capacity of Tregs in a dose-dependent manner. However, the production of cytokines secreted by Tregs and CD4+CD25- T cells was only inhibited at high concentrations of nilotinib exceeding the mean therapeutic serum concentrations of the drug in patients. Only high doses of nilotinib arrested both Tregs and CD4+CD25- T cells in the G0/G1 phase and down-regulated the expression of FoxP3 and GITR. In western blotting analysis, nilotinib did not show significant inhibitory effects on TCR signaling events in Tregs and CD4+CD25- T cells.
Conclusions: These findings indicate that nilotinib does not hamper the function of Tregs at clinical relevant doses, while long-term administration of nilotinib still needs to be investigated.
Figures
Similar articles
-
The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro.Int Immunol. 2007 Jun;19(6):785-99. doi: 10.1093/intimm/dxm047. Epub 2007 Jun 1. Int Immunol. 2007. PMID: 17545278
-
Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells.Br J Haematol. 2009 Jan;144(2):195-205. doi: 10.1111/j.1365-2141.2008.07433.x. Epub 2008 Nov 7. Br J Haematol. 2009. PMID: 19016717
-
Rapamycin, unlike cyclosporine A, enhances suppressive functions of in vitro-induced CD4+CD25+ Tregs.Nephrol Dial Transplant. 2010 Mar;25(3):710-7. doi: 10.1093/ndt/gfp586. Epub 2009 Nov 9. Nephrol Dial Transplant. 2010. PMID: 19903662
-
Signaling triggered by glucocorticoid-induced tumor necrosis factor receptor family-related gene: regulation at the interface between regulatory T cells and immune effector cells.Front Biosci. 2006 May 1;11:1448-65. doi: 10.2741/1895. Front Biosci. 2006. PMID: 16368528 Review.
-
Eras of designer Tregs: Harnessing synthetic biology for immune suppression.Immunol Rev. 2023 Nov;320(1):250-267. doi: 10.1111/imr.13254. Epub 2023 Jul 31. Immunol Rev. 2023. PMID: 37522861 Review.
Cited by
-
Nilotinib efficacy and safety as salvage treatment following imatinib intolerance and/or inefficacy in steroid refractory chronic graft-versus-host-disease (SR-cGVHD): a prospective, multicenter, phase II study on behalf of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC).Bone Marrow Transplant. 2023 Apr;58(4):401-406. doi: 10.1038/s41409-022-01898-x. Epub 2023 Jan 9. Bone Marrow Transplant. 2023. PMID: 36624161 Clinical Trial.
-
Nilotinib-Induced Elephantine Psoriasis In a Patient With Chronic Myeloid Leukemia: A Rare Case Report and Literature Review.Curr Ther Res Clin Exp. 2022 Jun 3;96:100676. doi: 10.1016/j.curtheres.2022.100676. eCollection 2022. Curr Ther Res Clin Exp. 2022. PMID: 35789635 Free PMC article.
-
Nilotinib: A Tyrosine Kinase Inhibitor Mediates Resistance to Intracellular Mycobacterium Via Regulating Autophagy.Cells. 2019 May 26;8(5):506. doi: 10.3390/cells8050506. Cells. 2019. PMID: 31130711 Free PMC article.
-
Differences in PD-1 expression on CD8+ T-cells in chronic myeloid leukemia patients according to disease phase and TKI medication.Cancer Immunol Immunother. 2020 Nov;69(11):2223-2232. doi: 10.1007/s00262-020-02617-5. Epub 2020 May 30. Cancer Immunol Immunother. 2020. PMID: 32474769 Free PMC article.
-
Activated naïve γδ T cells accelerate deep molecular response to BCR-ABL inhibitors in patients with chronic myeloid leukemia.Blood Cancer J. 2021 Nov 16;11(11):182. doi: 10.1038/s41408-021-00572-7. Blood Cancer J. 2021. PMID: 34785653 Free PMC article.
References
-
- Golemovic M, Verstovsek S, Giles F, Cortes J, Manshouri T, Manley PW, Mestan J, Dugan M, Alland L, Griffin JD, Arlinghaus RB, Sun T, Kantarjian H, Beran M. AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia. Clin Cancer Res. 2005;11:4941–7. doi: 10.1158/1078-0432.CCR-04-2601. - DOI - PubMed
-
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–51. doi: 10.1056/NEJMoa055104. - DOI - PubMed
-
- O'Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, Cowan-Jacob SW, Lee FY, Heinrich MC, Deininger MW, Druker BJ. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–5. doi: 10.1158/0008-5472.CAN-05-0259. - DOI - PubMed
-
- Verstovsek S, Golemovic M, Kantarjian H, Manshouri T, Estrov Z, Manley P, Sun T, Arlinghaus RB, Alland L, Dugan M, Cortes J, Giles F, Beran M. AMN107, a novel aminopyrimidine inhibitor of p190 Bcr-Abl activation and of in vitro proliferation of Philadelphia-positive acute lymphoblastic leukemia cells. Cancer. 2005;104:1230–6. doi: 10.1002/cncr.21299. - DOI - PubMed
-
- Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Neuberg D, Wright RD, Gilliland DG, Griffin JD. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–41. doi: 10.1016/j.ccr.2005.01.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

