Comparative in vitro study of the antimicrobial activities of different commercial antibiotic products for intravenous administration
- PMID: 20113478
- PMCID: PMC2830186
- DOI: 10.1186/1472-6904-10-3
Comparative in vitro study of the antimicrobial activities of different commercial antibiotic products for intravenous administration
Abstract
Background: The antimicrobial resistance is a global problem, probably due to the indiscriminate and irrational use of antibiotics, prescriptions for incorrect medicines or incorrect determinations of dose, route and/or duration. Another consideration is the uncertainty of patients receiving antibiotics about whether the quality of a generic medicine is equal to, greater than or less than its equivalent brand-name drug. The antibiotics behaviors must be evaluated in vitro and in vivo in order to confirm their suitability for therapeutic use.
Methods: The antimicrobial activities of Meropenem and Piperacillin/Tazobactam were studied by microbiological assays to determine their potencies (content), minimal inhibitory concentrations (MICs), critical concentrations and capacity to produce spontaneous drug-resistant mutants.
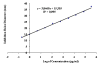
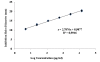
Results: With respect to potency (content) all the products fulfill USP requirements, so they should all be considered pharmaceutical equivalents. The MIC values of the samples evaluated (trade marks and generics) were the same for each strain tested, indicating that all products behaved similarly. The critical concentration values were very similar for all samples, and the ratios between the critical concentration of the standard and those of each sample were similar to the ratios of their specific antibiotic contents. Overall, therefore, the results showed no significant differences among samples. Finally, the production of spontaneous mutants did not differ significantly among the samples evaluated.
Conclusions: All the samples are pharmaceutical equivalents and the products can be used in antimicrobial therapy.
Figures






References
-
- World Health Organization. Communicable Disease Surveillance and Response Containing. Antimicrobial Resistance: Review of the Literature and Report of a WHO Workshop on the Development of a Global Strategy for the Containment of Antimicrobial Resistance. Geneva, Switzerland. 1999.
-
- Centers for disease control and prevention, food and drug administration, national institutes of health. A Public Health Action Plan to Combat Antimicrobial Resistance. http://www.cdc.gov/drugresistance/actionplan/index.htm
-
- Rodriguez CA, Zuluaga AF, Salazar BE, Agudelo M, Vesga O. Experimental comparison of 11 generic products (GP) of oxacillin (OXA) with the original compound (OC) in terms of concentration of active principle (CAP), in vitro activity, and in vivo efficacy, using the neutropenic murine thigh infection model (NMTIM) Abstr. A-1305. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Washington, D.C. 2004. p. 28.
-
- Zuluaga AF, Salazar BE, Loaiza SA, Agudelo M, Vesga O. Therapeutic equivalence (TE) with the original compound (OC) of 8 genetic products (GP) of Ampicillin (AMP) determined in the neutropenic murine thigh infection model (NMTIM) Abstr. E- 203344th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Washington, D.C. 2004.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

