Proteoglycans in host-pathogen interactions: molecular mechanisms and therapeutic implications
- PMID: 20113533
- PMCID: PMC4634875
- DOI: 10.1017/S1462399409001367
Proteoglycans in host-pathogen interactions: molecular mechanisms and therapeutic implications
Abstract
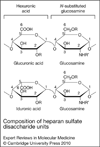
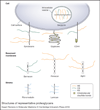
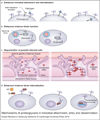
Many microbial pathogens subvert proteoglycans for their adhesion to host tissues, invasion of host cells, infection of neighbouring cells, dissemination into the systemic circulation, and evasion of host defence mechanisms. Where studied, specific virulence factors mediate these proteoglycan-pathogen interactions, which are thus thought to affect the onset, progression and outcome of infection. Proteoglycans are composites of glycosaminoglycan (GAG) chains attached covalently to specific core proteins. Proteoglycans are expressed ubiquitously on the cell surface, in intracellular compartments, and in the extracellular matrix. GAGs mediate the majority of ligand-binding activities of proteoglycans, and many microbial pathogens elaborate cell-surface and secreted factors that interact with GAGs. Some pathogens also modulate the expression and function of proteoglycans through known virulence factors. Several GAG-binding pathogens can no longer attach to and invade host cells whose GAG expression has been reduced by mutagenesis or enzymatic treatment. Furthermore, GAG antagonists have been shown to inhibit microbial attachment and host cell entry in vitro and reduce virulence in vivo. Together, these observations underscore the biological significance of proteoglycan-pathogen interactions in infectious diseases.
Figures

References
-
- Bernfield M, et al. Functions of cell surface heparan sulfate proteoglycans. Annual Review of Biochemistry. 1999;68:729–777. - PubMed
-
- Hartmann U, Maurer P. Proteoglycans in the nervous system–the quest for functional roles in vivo. Matrix Biology. 2001;20:23–35. - PubMed
-
- Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. Journal of Biological Chemistry. 2000;275:29923–29926. - PubMed
-
- Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB Journal. 2006;20:9–22. - PubMed
Further reading, resources and contacts
-
-
Shukla D, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. This paper elegantly showed that heparan sulfate (HS) modified by a subset of 3-O-sulfotransferase isoforms binds to HSV-1 gD and mediates viral entry. These data suggest that certain modifications might enable heparan sulfate proteoglycans (HSPGs) to function as both attachment and internalisation receptors for pathogens.
-
-
-
Reeves EP, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. This paper described a novel function of intracellular proteoglycans in modulating bacterial killing mechanisms of neutrophils. The study showed that upon neutrophil activation, a surge of K+ ions enter granules to compensate for the accumulation of anionic charge provided by the influx of reactive oxygen species. The rise in ionic strength activates cationic granule proteins, such as elastase and cathepsin G, by releasing them from the anionic proteoglycan matrix, enabling them to kill phagocytosed bacteria. These data show that proteoglycans can also modulate intracellular host defence mechanisms against pathogens.
-
-
-
Hayashida A, et al. Staphylococcus aureus beta-toxin induces acute lung injury through syndecan-1. American Journal of Pathology. 2009;174:509–518. This paper showed for the first time in vivo that a bacterial virulence factor causes inflammatory tissue damage by dysregulating the capacity of syndecan-1 to moderate neutrophil infiltration.
-
-
-
Baleux F, et al. A synthetic CD4-heparan sulfate glycoconjugate inhibits CCR5 and CXCR4 HIV-1 attachment and entry. Nature Chemical Biology. 2009;5:743–748. This paper described a chemical approach to synthesise a CD 4-mimetic peptide linked to an HS dodecasaccharide. The study showed that the linkage between the CD4 mimetic and HS derivative provides strong cooperative effects, resulting in low-nanomolar antiviral activity against both CCR5- and CXCR4-tropic HIV-1 strains. The study demonstrates the feasibility of and efficacy of a new type of inhibitor, which has the unique ability to target simultaneously two critical and highly conserved regions of gp120.
-
-
-
Vivès RR, Lortat-Jacob H, Fender P. Heparan sulphate proteoglycans and viral vectors: ally or foe? Current Gene Therapy. 2006;6:35–44. This review provides an overview on the multiple roles of HSPGs during viral infection, with a special focus on viruses used as gene delivery vectors. It evaluates various strategies that have been developed to potentiate the advantages or to overcome the drawbacks resulting from viral vector interaction with HS.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

