Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry
- PMID: 20113874
- PMCID: PMC4199380
- DOI: 10.1016/j.alcohol.2009.09.035
Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry
Abstract
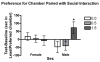
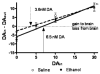
Alcohol use increases across adolescence and is a concern in the United States. In humans, males and females consume different amounts of alcohol depending on the age of initiation, and the long-term consequences of early ethanol consumption are not readily understood. The purpose of our work was to better understand the immediate and long-term impact of ethanol exposure during adolescence and the effects it can have on behavior and dopaminergic responsivity. We have assessed sex differences in voluntary ethanol consumption during adolescence and adulthood and the influence of binge ethanol exposure during adolescence. We have observed that males are sensitive to passive social influences that mediate voluntary ethanol consumption, and early ethanol exposure induces long-term changes in responsivity to ethanol in adulthood. Exposure to moderate doses of ethanol during adolescence produced alterations in dopamine in the nucleus accumbens septi during adolescence and later in adulthood. Taken together, all of these data indicate that the adolescent brain is sensitive to the impact of early ethanol exposure during this critical developmental period.
2010 Elsevier Inc. All rights reserved.
Figures

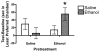
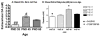
References
-
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. - PubMed
-
- Badanich KA, Maldonado AM, Kirstein CL. Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res. 2007;31(5):895–900. - PubMed
-
- Bates ME, Labouvie EW. Adolescent risk factors and the prediction of persistent alcohol and drug use into adulthood. Alcohol Clin Exp Res. 1997;21(5):944–950. - PubMed
-
- Beck KH, Thombs DL, Summons TG. The social context of drinking scales: construct validation and relationship to indicants of abuse in an adolescent population. Addict Behav. 1993;18(2):159–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

