A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria
- PMID: 20114026
- PMCID: PMC2831478
- DOI: 10.1016/j.chom.2009.12.007
A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria
Abstract
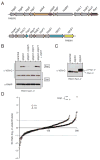
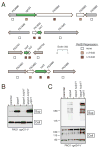
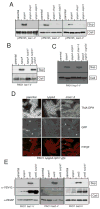
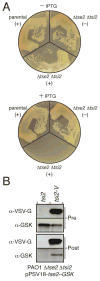
The functional spectrum of a secretion system is defined by its substrates. Here we analyzed the secretomes of Pseudomonas aeruginosa mutants altered in regulation of the Hcp Secretion Island-I-encoded type VI secretion system (H1-T6SS). We identified three substrates of this system, proteins Tse1-3 (type six exported 1-3), which are coregulated with the secretory apparatus and secreted under tight posttranslational control. The Tse2 protein was found to be the toxin component of a toxin-immunity system and to arrest the growth of prokaryotic and eukaryotic cells when expressed intracellularly. In contrast, secreted Tse2 had no effect on eukaryotic cells; however, it provided a major growth advantage for P. aeruginosa strains, relative to those lacking immunity, in a manner dependent on cell contact and the H1-T6SS. This demonstration that the T6SS targets a toxin to bacteria helps reconcile the structural and evolutionary relationship between the T6SS and the bacteriophage tail and spike.
2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion--mycobacteria show the way. Nat Rev Microbiol. 2007;5:883–891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

