Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway
- PMID: 20114038
- PMCID: PMC2836484
- DOI: 10.1016/j.brainres.2010.01.046
Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway
Abstract
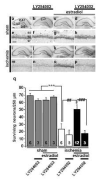
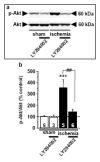
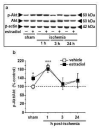
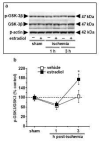
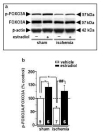
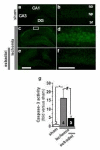
Global ischemia arising during cardiac arrest or cardiac surgery causes highly selective, delayed death of hippocampal CA1 neurons. Exogenous estradiol ameliorates global ischemia-induced neuronal death and cognitive impairment in male and female rodents. However, the molecular mechanisms by which a single acute injection of estradiol administered after the ischemic event intervenes in global ischemia-induced apoptotic cell death are unclear. Here we show that acute estradiol acts via the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling cascade to protect CA1 neurons in ovariectomized female rats. We demonstrate that global ischemia promotes early activation of glycogen synthase kinase-3beta (GSK3beta) and forkhead transcription factor of the O class (FOXO)3A, known Akt targets that are related to cell survival, and activation of caspase-3. Estradiol prevents ischemia-induced dephosphorylation and activation of GSK3beta and FOXO3A, and the caspase death cascade. These findings support a model whereby estradiol acts by activation of PI3K/Akt signaling to promote neuronal survival in the face of global ischemia.
2010 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia.Endocrinology. 2007 Mar;148(3):1131-43. doi: 10.1210/en.2006-1137. Epub 2006 Nov 30. Endocrinology. 2007. PMID: 17138646 Free PMC article.
-
Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia.Neurol Res. 2009 Nov;31(9):947-58. doi: 10.1179/174313209X382449. Epub 2009 Feb 6. Neurol Res. 2009. PMID: 19203442
-
Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats.J Cereb Blood Flow Metab. 2006 Dec;26(12):1479-89. doi: 10.1038/sj.jcbfm.9600303. Epub 2006 Mar 15. J Cereb Blood Flow Metab. 2006. PMID: 16538228
-
Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection.Steroids. 2009 Jul;74(7):555-61. doi: 10.1016/j.steroids.2009.01.003. Epub 2009 Jan 20. Steroids. 2009. PMID: 19428444 Free PMC article. Review.
-
Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: translational implications.Front Neuroendocrinol. 2011 Aug;32(3):336-52. doi: 10.1016/j.yfrne.2010.12.005. Epub 2010 Dec 14. Front Neuroendocrinol. 2011. PMID: 21163293 Free PMC article. Review.
Cited by
-
The role of Foxo3a in neuron-mediated cognitive impairment.Front Mol Neurosci. 2024 Jun 19;17:1424561. doi: 10.3389/fnmol.2024.1424561. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38962803 Free PMC article. Review.
-
Estradiol attenuates ischemia-induced death of hippocampal neurons and enhances synaptic transmission in aged, long-term hormone-deprived female rats.PLoS One. 2012;7(6):e38018. doi: 10.1371/journal.pone.0038018. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675505 Free PMC article.
-
Isoflavone-Enriched Soybean Leaves (Glycine Max) Alleviate Cognitive Impairment Induced by Ovariectomy and Modulate PI3K/Akt Signaling in the Hippocampus of C57BL6 Mice.Nutrients. 2022 Nov 10;14(22):4753. doi: 10.3390/nu14224753. Nutrients. 2022. PMID: 36432439 Free PMC article.
-
Sex differences in acute cardiovascular care: a review and needs assessment.Cardiovasc Res. 2022 Feb 21;118(3):667-685. doi: 10.1093/cvr/cvab063. Cardiovasc Res. 2022. PMID: 33734314 Free PMC article. Review.
-
Single dose of 17β-estradiol provides transient neuroprotection in female juvenile mice after cardiac-arrest and cardiopulmonary resuscitation.Neurochem Int. 2019 Jul;127:80-86. doi: 10.1016/j.neuint.2018.11.013. Epub 2018 Nov 22. Neurochem Int. 2019. PMID: 30471325 Free PMC article.
References
-
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol. Res. 2005;52:119–132. - PubMed
-
- Amstad PA, Yu G, Johnson GL, Lee BW, Dhawan S, Phelps DJ. Detection of caspase activation in situ by fluorochrome-labeled caspase inhibitors. Biotechniques. 2001;31:608–10. 612, 614. passim. - PubMed
-
- Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp. Cell Res. 2000;259:308–313. - PubMed
-
- Behl C. Oestrogen as a neuroprotective hormone. Nature Reviews. 2002;3:433–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

