Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors
- PMID: 20114055
- PMCID: PMC2849849
- DOI: 10.1016/j.neuropharm.2010.01.013
Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors
Abstract
Mammalian brain expresses multiple nicotinic acetylcholine receptor (nAChR) subtypes that differ in subunit composition, sites of expression and pharmacological and functional properties. Among known subtypes of receptors, alpha 4 beta 2* and alpha 6 beta 2*-nAChR have the highest affinity for nicotine (where * indicates possibility of other subunits). The alpha 4 beta 2*-nAChRs are widely distributed, while alpha 6 beta 2*-nAChR are restricted to a few regions. Both subtypes modulate release of dopamine from the dopaminergic neurons of the mesoaccumbens pathway thought to be essential for reward and addiction. alpha 4 beta 2*-nAChR also modulate GABA release in these areas. Identification of selective compounds would facilitate study of nAChR subtypes. An improved understanding of the role of nAChR subtypes may help in developing more effective smoking cessation aids with fewer side effects than current therapeutics. We have screened a series of nicotinic compounds that vary in the distance between the pyridine and the cationic center, in steric bulk, and in flexibility of the molecule. These compounds were screened using membrane binding and synaptosomal function assays, or recordings from GH4C1 cells expressing h alpha 7, to determine affinity, potency and efficacy at four subtypes of nAChRs found in brain, alpha 4 beta 2*, alpha 6 beta 2*, alpha 7 and alpha 3 beta 4*. In addition, physiological assays in gain-of-function mutant mice were used to assess in vivo activity at alpha 4 beta 2* and alpha 6 beta 2*-nAChRs. This approach has identified several compounds with agonist or partial agonist activity that display improved selectivity for alpha 6 beta 2*-nAChR.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures




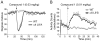
References
-
- Bencherif M, Lovette ME, Fowler KW, Arrington S, Reeves L, Caldwell WS, Lippiello PM. RJR-2403: a nicotinic agonist with CNS selectivity I. In vitro characterization. J Pharmacol Exp Ther. 1996;279:1413–21. - PubMed
-
- Bhatti BS, Strachan JP, Breining SR, Miller CH, Tahiri P, Crooks PA, Deo N, Day CS, Caldwell WS. Synthesis of 2-(pyridin-3-yl)-1-azabicyclo[3.2.2]nonane, 2-(pyridin-3-yl)-1-azabicyclo[2.2.2]octane, and 2-(pyridin-3-yl)-1-azabicyclo[3.2.1]octane, a class of potent nicotinic acetylcholine receptor-ligands. J Org Chem. 2008;73:3497–507. - PubMed
-
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

