Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind
- PMID: 20114075
- PMCID: PMC2874108
- DOI: 10.1016/j.nbd.2010.01.008
Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind
Abstract
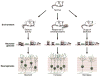
In mature, differentiated neurons in the central nervous system (CNS), epigenetic mechanisms--including DNA methylation, histone modification, and regulatory noncoding RNAs--play critical roles in encoding experience and environmental stimuli into stable, behaviorally meaningful changes in gene expression. For example, epigenetic changes in mature hippocampal neurons have been implicated in learning and memory and in a variety of neuropsychiatric disorders, including depression. With all the recent (and warranted) attention given to epigenetic modifications in mature neurons, it is easy to forget that epigenetic mechanisms were initially described for their ability to promote differentiation and drive cell fate in embryonic and early postnatal development, including neurogenesis. Given the discovery of ongoing neurogenesis in the adult brain and the intriguing links among adult hippocampal neurogenesis, hippocampal function, and neuropsychiatric disorders, it is timely to complement the ongoing discussions on the role of epigenetics in mature neurons with a review on what is currently known about the role of epigenetics in adult hippocampal neurogenesis. The process of adult hippocampal neurogenesis is complex, with neural stem cells (NSCs) giving rise to fate-restricted progenitors and eventually mature dentate gyrus granule cells. Notably, neurogenesis occurs within an increasingly well-defined "neurogenic niche", where mature cellular elements like vasculature, astrocytes, and neurons release signals that can dynamically regulate neurogenesis. Here we review the evidence that key stages and aspects of adult neurogenesis are driven by epigenetic mechanisms. We discuss the intrinsic changes occurring within NSCs and their progeny that are critical for neurogenesis. We also discuss how extrinsic changes occurring in cellular components in the niche can result in altered neurogenesis. Finally we describe the potential relevance of epigenetics for understanding the relationship between hippocampal neurogenesis in neuropsychiatric disorders. We propose that a more thorough understanding of the molecular and genetic mechanisms that control the complex process of neurogenesis, including the proliferation and differentiation of NSCs, will lead to novel therapeutics for the treatment of neuropsychiatric disorders.
(c) 2010 Elsevier Inc.
Figures


References
-
- Abrous DN, et al. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–69. - PubMed
-
- Alarcon JM, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–59. - PubMed
-
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

