Activation of progestin receptors in female reproductive behavior: Interactions with neurotransmitters
- PMID: 20116396
- PMCID: PMC2849835
- DOI: 10.1016/j.yfrne.2010.01.002
Activation of progestin receptors in female reproductive behavior: Interactions with neurotransmitters
Abstract
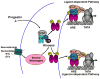
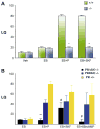
The steroid hormone, progesterone (P), modulates neuroendocrine functions in the central nervous system resulting in alterations in physiology and reproductive behavior in female mammals. A wide body of evidence indicates that these neural effects of P are predominantly mediated via their intracellular progestin receptors (PRs) functioning as "ligand-dependent" transcription factors in the steroid-sensitive neurons regulating genes and genomic networks. In addition to P, intracellular PRs can be activated by neurotransmitters, growth factors and cyclic nucleotides in a ligand-independent manner via crosstalk and convergence of pathways. Furthermore, recent studies indicate that rapid signaling events associated with membrane PRs and/or extra-nuclear, cytoplasmic PRs converge with classical PR activated pathways in neuroendocrine regulation of female reproductive behavior. The molecular mechanisms, by which multiple signaling pathways converge on PRs to modulate PR-dependent female reproductive behavior, are discussed in this review.
Figures



Similar articles
-
Ligand-independent activation of progestin receptors in sexual receptivity.Horm Behav. 2001 Sep;40(2):183-90. doi: 10.1006/hbeh.2001.1687. Horm Behav. 2001. PMID: 11534980 Review.
-
Neural progestin receptors and female sexual behavior.Neuroendocrinology. 2012;96(2):152-61. doi: 10.1159/000338668. Epub 2012 Sep 14. Neuroendocrinology. 2012. PMID: 22538437 Free PMC article. Review.
-
Progesterone signaling mechanisms in brain and behavior.Front Endocrinol (Lausanne). 2012 Jan 30;3:7. doi: 10.3389/fendo.2012.00007. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22649404 Free PMC article.
-
Signaling mechanisms in progesterone-neurotransmitter interactions.Neuroscience. 2006;138(3):773-81. doi: 10.1016/j.neuroscience.2005.07.034. Epub 2005 Nov 28. Neuroscience. 2006. PMID: 16310962 Review.
-
Steroid hormone action in the brain: cross-talk between signalling pathways.J Neuroendocrinol. 2009 Mar;21(4):243-7. doi: 10.1111/j.1365-2826.2009.01844.x. J Neuroendocrinol. 2009. PMID: 19187467 Review.
Cited by
-
Missing and Possible Link between Neuroendocrine Factors, Neuropsychiatric Disorders, and Microglia.Front Integr Neurosci. 2013 Jul 15;7:53. doi: 10.3389/fnint.2013.00053. eCollection 2013. Front Integr Neurosci. 2013. PMID: 23874274 Free PMC article.
-
An antiprogestin, CDB4124, blocks progesterone's attenuation of the negative effects of a mild stress on sexual behavior.Behav Brain Res. 2013 Mar 1;240:21-5. doi: 10.1016/j.bbr.2012.11.002. Epub 2012 Nov 12. Behav Brain Res. 2013. PMID: 23153933 Free PMC article.
-
Mechanisms responsible for progesterone's protection against lordosis-inhibiting effects of restraint I. Role of progesterone receptors.Horm Behav. 2011 Jul;60(2):219-25. doi: 10.1016/j.yhbeh.2011.05.006. Epub 2011 May 20. Horm Behav. 2011. PMID: 21635894 Free PMC article.
-
Dose-dependent effects of the antiprogestin, RU486, on sexual behavior of naturally cycling Fischer rats.Behav Brain Res. 2015 Apr 1;282:95-102. doi: 10.1016/j.bbr.2015.01.004. Epub 2015 Jan 12. Behav Brain Res. 2015. PMID: 25591479 Free PMC article.
-
RU486 blocks effects of allopregnanolone on the response to restraint stress.Pharmacol Biochem Behav. 2013 Jan;103(3):568-72. doi: 10.1016/j.pbb.2012.09.024. Epub 2012 Oct 6. Pharmacol Biochem Behav. 2013. PMID: 23046854 Free PMC article.
References
-
- Acosta-Martinez M, Etgen AM. The role of delta-opioid receptors in the facilitation of lordosis behavior. Behav Brain Res. 2002;136:93–102. - PubMed
-
- Acosta-Martínez M, Gonzalez-Flores O, Etgen AM. The role of progestin receptors and the mitogen-activated protein kinase pathway in delta opioid receptor facilitation of female reproductive behaviors. Horm Behav. 2006;49:458–462. - PubMed
-
- Alkhalaf M, Murphy LC. Regulation of c-jun and jun-B by progestins in T47-D human breast cancer cells. Mol Endo. 1992;6:1625–1633. - PubMed
-
- An BS, Selva DM, Hammond GL, Rivero-Muller A, Rahman N, Leung PCK. Steroid receptor coactivator-3 is required for progesterone receptor transactivation of target genes in response to gonadotropin-releasing hormone treatment of pituitary cells. J Biol Chem. 2006;281:20817–20824. - PubMed
-
- Apostolakis EM, Ramamurphy M, Zhou D, Oñate S, O’Malley BW. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol. 2002;16:1511–1523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

