Use of glycol chitosan modified by 5beta-cholanic acid nanoparticles for the sustained release of proteins during murine embryonic limb skeletogenesis
- PMID: 20116406
- PMCID: PMC3363971
- DOI: 10.1016/j.jconrel.2010.01.021
Use of glycol chitosan modified by 5beta-cholanic acid nanoparticles for the sustained release of proteins during murine embryonic limb skeletogenesis
Abstract
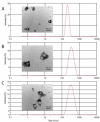
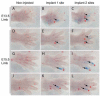
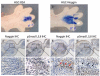
Murine embryonic limb cultures have invaluable roles in studying skeletogenesis. Substance delivery is an underdeveloped area in developmental biology that has primarily relied on Affi-Gel-Blue-agarose-beads. However, the lack of information about the efficiency of agarose-bead loading and release and difficulties for a single-bead implantation represent significant limitations. We optimized the use of glycol chitosan-5beta-cholanic acid conjugates (HGC) as a novel protein delivery system in mouse embryonic limbs. To this purpose, we loaded HGC either with recombinant Noggin, or bovine serum albumin (BSA). The size, morphology and stability of the protein-loaded-HGC were determined by transmission electron microscopy and dynamic-light-scattering. HGC-BSA and HGC-Noggin loading efficiencies were 80-90%. Time-course study revealed that Noggin and BSA were 80-90% released after 48 h. We developed several techniques to implant protein-loaded-HGC into murine embryonic joints from embryonic age E13.5 to E15.5, including a micro-injection system dispensing nanoliters. HGC did not interfere with skeletogenesis. Using CBR-3BA staining, we detected HGC nanoparticles within implanted tissues. Furthermore, a sustained release of BSA and Noggin was demonstrated in HGC-BSA and HGC-Noggin injected regions. HGC-released Noggin was biologically active in blocking the BMP signaling in in vitro mesenchyme limb micromasses as well as in ex-vivo limb cultures. Results reveal that HGC is a valuable protein-delivery system in developmental biology.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Self-assembled nanoparticles containing hydrophobically modified glycol chitosan for gene delivery.J Control Release. 2005 Mar 2;103(1):235-43. doi: 10.1016/j.jconrel.2004.11.033. Epub 2005 Jan 15. J Control Release. 2005. PMID: 15710514
-
Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel.J Control Release. 2006 Mar 10;111(1-2):228-34. doi: 10.1016/j.jconrel.2005.12.013. Epub 2006 Feb 3. J Control Release. 2006. PMID: 16458988
-
Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy.J Control Release. 2008 May 8;127(3):208-18. doi: 10.1016/j.jconrel.2008.01.013. Epub 2008 Feb 7. J Control Release. 2008. PMID: 18336946
-
Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy.Biomaterials. 2008 Apr;29(12):1920-30. doi: 10.1016/j.biomaterials.2007.12.038. Biomaterials. 2008. PMID: 18289669
-
Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles.J Control Release. 2008 May 22;128(1):23-31. doi: 10.1016/j.jconrel.2008.02.003. Epub 2008 Feb 20. J Control Release. 2008. PMID: 18374444
Cited by
-
Chitosan Based Self-Assembled Nanoparticles in Drug Delivery.Polymers (Basel). 2018 Feb 26;10(3):235. doi: 10.3390/polym10030235. Polymers (Basel). 2018. PMID: 30966270 Free PMC article. Review.
-
TGF-β type II receptor/MCP-5 axis: at the crossroad between joint and growth plate development.Dev Cell. 2012 Jul 17;23(1):71-81. doi: 10.1016/j.devcel.2012.05.004. Dev Cell. 2012. PMID: 22814601 Free PMC article.
-
The Application of Chitosan Nanostructures in Stomatology.Molecules. 2021 Oct 19;26(20):6315. doi: 10.3390/molecules26206315. Molecules. 2021. PMID: 34684896 Free PMC article. Review.
-
High molecular weight chitosan derivative polymeric micelles encapsulating superparamagnetic iron oxide for tumor-targeted magnetic resonance imaging.Int J Nanomedicine. 2015 Feb 5;10:1155-72. doi: 10.2147/IJN.S70022. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25709439 Free PMC article.
-
Oral Soft Tissue Regeneration Using Nano Controlled System Inducing Sequential Release of Trichloroacetic Acid and Epidermal Growth Factor.Tissue Eng Regen Med. 2020 Feb;17(1):91-103. doi: 10.1007/s13770-019-00232-9. Epub 2020 Jan 22. Tissue Eng Regen Med. 2020. PMID: 31970697 Free PMC article.
References
-
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69(2):144–155. - PubMed
-
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280(5368):1455–1457. - PubMed
-
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368(6472):639–643. - PubMed
-
- Hayek A, Culler FL, Beattie GM, Lopez AD, Cuevas P, Baird A. An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem Biophys Res Commun. 1987;147(2):876–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

