Avian influenza viral nucleocapsid and hemagglutinin proteins induce chicken CD8+ memory T lymphocytes
- PMID: 20116819
- PMCID: PMC7111969
- DOI: 10.1016/j.virol.2009.12.029
Avian influenza viral nucleocapsid and hemagglutinin proteins induce chicken CD8+ memory T lymphocytes
Abstract
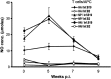
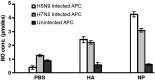
The avian influenza viruses (AIVs) can be highly contagious to poultry and a zoonotic threat to humans. Since the memory CD8(+) T lymphocyte responses in chickens to AIV proteins have not been defined, these responses to H5N9 AIV hemagglutinin (HA) and nucleocapsid (NP) proteins were evaluated by ex vivo stimulation with virus infected non-professional antigen presenting cells. Secretion of IFNgamma by activated T lymphocytes was evaluated through macrophage induction of nitric oxide. AIV specific, MHC-I restricted memory CD8(+) T lymphocyte responses to NP and HA were observed 3 to 9 weeks post-inoculation (p.i.). The responses specific to NP were greater than those to HA with maximum responses being observed at 5 weeks p.i. followed by a decline to weakly detectable levels by 9 weeks p.i. The cross-reaction of T lymphocytes to a heterologous H7N2 AIV strain demonstrated their ability to respond to a broader range of AIV.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Non-replicating adenovirus vectors expressing avian influenza virus hemagglutinin and nucleocapsid proteins induce chicken specific effector, memory and effector memory CD8(+) T lymphocytes.Virology. 2010 Sep 15;405(1):62-9. doi: 10.1016/j.virol.2010.05.002. Epub 2010 Jun 16. Virology. 2010. PMID: 20557918 Free PMC article.
-
Consecutive natural influenza a virus infections in sentinel mallards in the evident absence of subtype-specific hemagglutination inhibiting antibodies.Transbound Emerg Dis. 2013 Oct;60(5):395-402. doi: 10.1111/j.1865-1682.2012.01357.x. Epub 2012 Jul 22. Transbound Emerg Dis. 2013. PMID: 22816511
-
Seasonal influenza vaccination is the strongest correlate of cross-reactive antibody responses in migratory bird handlers.mBio. 2014 Dec 9;5(6):e02107. doi: 10.1128/mBio.02107-14. mBio. 2014. PMID: 25491354 Free PMC article.
-
Immune responses to avian influenza viruses in chickens.Virology. 2025 Feb;603:110405. doi: 10.1016/j.virol.2025.110405. Epub 2025 Jan 16. Virology. 2025. PMID: 39837219 Review.
-
Avian Influenza Virus and DIVA Strategies.Viral Immunol. 2016 May;29(4):198-211. doi: 10.1089/vim.2015.0127. Epub 2016 Feb 22. Viral Immunol. 2016. PMID: 26900835 Review.
Cited by
-
Towards a universal vaccine for avian influenza: protective efficacy of modified Vaccinia virus Ankara and Adenovirus vaccines expressing conserved influenza antigens in chickens challenged with low pathogenic avian influenza virus.Vaccine. 2013 Jan 11;31(4):670-5. doi: 10.1016/j.vaccine.2012.11.047. Epub 2012 Nov 28. Vaccine. 2013. PMID: 23200938 Free PMC article.
-
Association of the chicken MHC B haplotypes with resistance to avian coronavirus.Dev Comp Immunol. 2013 Apr;39(4):430-7. doi: 10.1016/j.dci.2012.10.006. Epub 2012 Nov 23. Dev Comp Immunol. 2013. PMID: 23178407 Free PMC article.
-
Non-replicating adenovirus vectors expressing avian influenza virus hemagglutinin and nucleocapsid proteins induce chicken specific effector, memory and effector memory CD8(+) T lymphocytes.Virology. 2010 Sep 15;405(1):62-9. doi: 10.1016/j.virol.2010.05.002. Epub 2010 Jun 16. Virology. 2010. PMID: 20557918 Free PMC article.
-
Quantification and phenotypic characterisation of peripheral IFN-γ producing leucocytes in chickens vaccinated against Newcastle disease.Vet Immunol Immunopathol. 2017 Dec;193-194:18-28. doi: 10.1016/j.vetimm.2017.10.001. Epub 2017 Oct 10. Vet Immunol Immunopathol. 2017. PMID: 29129224 Free PMC article.
-
Identification of novel avian influenza virus derived CD8+ T-cell epitopes.PLoS One. 2012;7(2):e31953. doi: 10.1371/journal.pone.0031953. Epub 2012 Feb 23. PLoS One. 2012. PMID: 22384112 Free PMC article.
References
-
- Alexander D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000;74(1-2):3–13. - PubMed
-
- Babakir-Mina M., Dimonte S., Perno C.F., Ciotti M. Origin of the 2009 Mexico influenza virus: a comparative phylogenetic analysis of the principal external antigens and matrix protein. Arch. Virol. 2009;154(8):1349–1352. - PubMed
-
- Beard C.W. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. In: Purchase L.H.A.H.G., Domermuth C.H., Pearson J.E., editors. 3rd ed. Kendall/Hunt Publishing Co; Dubuque, IA: 1989.
-
- Bohls R.L., Smith R., Ferro P.J., Silvy N.J., Li Z., Collisson E.W. The use of flow cytometry to discriminate avian lymphocytes from contaminating thrombocytes. Dev. Comp. Immunol. 2006;30(9):843–850. - PubMed
-
- Busch D.H., Pamer E.G. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J. Immunol. 1998;160(9):4441–4448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

