Pathogenesis of hepatitis B virus infection
- PMID: 20116937
- PMCID: PMC2888709
- DOI: 10.1016/j.patbio.2009.11.001
Pathogenesis of hepatitis B virus infection
Abstract
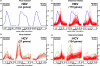
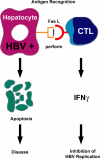
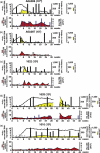
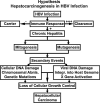
The adaptive immune response is thought to be responsible for viral clearance and disease pathogenesis during hepatitis B virus infection. It is generally acknowledged that the humoral antibody response contributes to the clearance of circulating virus particles and the prevention of viral spread within the host while the cellular immune response eliminates infected cells. The T cell response to the hepatitis B virus (HBV) is vigorous, polyclonal and multispecific in acutely infected patients who successfully clear the virus and relatively weak and narrowly focussed in chronically infected patients, suggesting that clearance of HBV is T cell dependent. The pathogenetic and antiviral potential of the cytotoxic T lymphocyte (CTL) response to HBV has been proven by the induction of a severe necroinflammatory liver disease following the adoptive transfer of HBsAg specific CTL into HBV transgenic mice. Remarkably, the CTLs also purge HBV replicative intermediates from the liver by secreting type 1 inflammatory cytokines thereby limiting virus spread to uninfected cells and reducing the degree of immunopathology required to terminate the infection. Persistent HBV infection is characterized by a weak adaptive immune response, thought to be due to inefficient CD4+ T cell priming early in the infection and subsequent development of a quantitatively and qualitatively ineffective CD8+ T cell response. Other factors that could contribute to viral persistence are immunological tolerance, mutational epitope inactivation, T cell receptor antagonism, incomplete down-regulation of viral replication and infection of immunologically privileged tissues. However, these pathways become apparent only in the setting of an ineffective immune response, which is, therefore, the fundamental underlying cause. Persistent infection is characterized by chronic liver cell injury, regeneration, inflammation, widespread DNA damage and insertional deregulation of cellular growth control genes, which, collectively, lead to cirrhosis of the liver and hepatocellular carcinoma.
Copyright 2009 Elsevier Masson SAS. All rights reserved.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

