Protein histidine kinases: assembly of active sites and their regulation in signaling pathways
- PMID: 20117042
- PMCID: PMC2847664
- DOI: 10.1016/j.mib.2009.12.013
Protein histidine kinases: assembly of active sites and their regulation in signaling pathways
Abstract
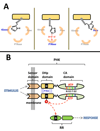
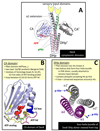
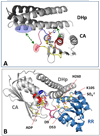
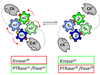
Protein histidine kinases (PHKs) function in Two Component Signaling pathways utilized extensively by bacteria and archaea. Many PHKs participate in three distinct, but interrelated signaling reactions: autophoshorylation, phosphotransfer (to a partner Response Regulator (RR) protein), and dephosphorylation of this RR. Detailed biochemical and structural characterization of several PHKs has revealed how the domains of these proteins can interact to assemble the three active sites that promote the necessary chemistry and how these domain interactions might be regulated in response to sensory input: the relative orientation of helices in the PHK dimerization domain can reorient, via cogwheeling (rotation) and kinking (bending), to effect changes in PHK activities that probably involve sequestration/release of the PHK catalytic domain by the dimerization domain.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Diversity of structure and function of response regulator output domains.Curr Opin Microbiol. 2010 Apr;13(2):150-9. doi: 10.1016/j.mib.2010.01.005. Epub 2010 Mar 11. Curr Opin Microbiol. 2010. PMID: 20226724 Free PMC article. Review.
-
Sensor domains of two-component regulatory systems.Curr Opin Microbiol. 2010 Apr;13(2):116-23. doi: 10.1016/j.mib.2010.01.016. Epub 2010 Mar 10. Curr Opin Microbiol. 2010. PMID: 20223701 Free PMC article. Review.
-
Common extracellular sensory domains in transmembrane receptors for diverse signal transduction pathways in bacteria and archaea.J Bacteriol. 2003 Jan;185(1):285-94. doi: 10.1128/JB.185.1.285-294.2003. J Bacteriol. 2003. PMID: 12486065 Free PMC article.
-
Protein disulfides and protein disulfide oxidoreductases in hyperthermophiles.FEBS J. 2006 Sep;273(18):4170-85. doi: 10.1111/j.1742-4658.2006.05421.x. Epub 2006 Aug 23. FEBS J. 2006. PMID: 16930136 Review.
-
Phyletic Distribution and Lineage-Specific Domain Architectures of Archaeal Two-Component Signal Transduction Systems.J Bacteriol. 2018 Mar 12;200(7):e00681-17. doi: 10.1128/JB.00681-17. Print 2018 Apr 1. J Bacteriol. 2018. PMID: 29263101 Free PMC article.
Cited by
-
Chemoproteomic Approaches for Unraveling Prokaryotic Biology.Isr J Chem. 2023 Mar;63(3-4):e202200076. doi: 10.1002/ijch.202200076. Epub 2023 Feb 28. Isr J Chem. 2023. PMID: 37842282 Free PMC article.
-
An intramembrane sensory circuit monitors sortase A-mediated processing of streptococcal adhesins.Sci Signal. 2019 May 7;12(580):eaas9941. doi: 10.1126/scisignal.aas9941. Sci Signal. 2019. PMID: 31064885 Free PMC article.
-
Stress-induced remodeling of the bacterial proteome.Curr Biol. 2014 May 19;24(10):R424-34. doi: 10.1016/j.cub.2014.03.023. Curr Biol. 2014. PMID: 24845675 Free PMC article. Review.
-
A two-component system regulates gene expression of the type IX secretion component proteins via an ECF sigma factor.Sci Rep. 2016 Mar 21;6:23288. doi: 10.1038/srep23288. Sci Rep. 2016. PMID: 26996145 Free PMC article.
-
Activation of ATP binding for the autophosphorylation of DosS, a Mycobacterium tuberculosis histidine kinase lacking an ATP lid motif.J Biol Chem. 2013 May 3;288(18):12437-47. doi: 10.1074/jbc.M112.442467. Epub 2013 Mar 13. J Biol Chem. 2013. PMID: 23486471 Free PMC article.
References
-
- Hoch JA, Silhavy TJ. Two-Component Signal Transduction. Washington, DC: ASM Press; 1995.
-
-
Gao R, Stock AM. Biological Insights from Structures of Two-Component Proteins. Ann Rev Microbiol. 2009;63:133–154. Both PHK afficianados and newcomers will appreciate this well written and up-to-date review.
-
-
- Saito H. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem Rev. 2001;101:2497–2509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

