Protein histidine kinases: assembly of active sites and their regulation in signaling pathways
- PMID: 20117042
- PMCID: PMC2847664
- DOI: 10.1016/j.mib.2009.12.013
Protein histidine kinases: assembly of active sites and their regulation in signaling pathways
Abstract
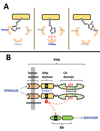
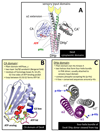
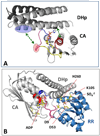
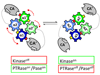
Protein histidine kinases (PHKs) function in Two Component Signaling pathways utilized extensively by bacteria and archaea. Many PHKs participate in three distinct, but interrelated signaling reactions: autophoshorylation, phosphotransfer (to a partner Response Regulator (RR) protein), and dephosphorylation of this RR. Detailed biochemical and structural characterization of several PHKs has revealed how the domains of these proteins can interact to assemble the three active sites that promote the necessary chemistry and how these domain interactions might be regulated in response to sensory input: the relative orientation of helices in the PHK dimerization domain can reorient, via cogwheeling (rotation) and kinking (bending), to effect changes in PHK activities that probably involve sequestration/release of the PHK catalytic domain by the dimerization domain.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Hoch JA, Silhavy TJ. Two-Component Signal Transduction. Washington, DC: ASM Press; 1995.
-
-
Gao R, Stock AM. Biological Insights from Structures of Two-Component Proteins. Ann Rev Microbiol. 2009;63:133–154. Both PHK afficianados and newcomers will appreciate this well written and up-to-date review.
-
-
- Saito H. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem Rev. 2001;101:2497–2509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

