Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity
- PMID: 20117110
- PMCID: PMC3049264
- DOI: 10.1053/j.gastro.2010.01.044
Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity
Abstract
Background & aims: Cholecystokinin (CCK) is a satiation peptide released during meals in response to lipid intake; it regulates pancreatic digestive enzymes that are required for absorption of nutrients. We proposed that mice with a disruption in the CCK gene (CCK knockout [CCK-KO] mice) that were fed a diet of 20% butter fat would have altered fat metabolism.
Methods: We used quantitative magnetic resonance imaging to determine body composition and monitored food intake of CCK-KO mice using an automated measurement system. Intestinal fat absorption and energy expenditure were determined using a noninvasive assessment of intestinal fat absorption and an open circuit calorimeter, respectively.
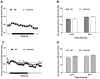
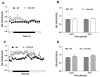
Results: After consuming a high-fat diet for 10 weeks, CCK-KO mice had reduced body weight gain and body fat mass and enlarged adipocytes, despite the same level of food intake as wild-type mice. CCK-KO mice also had defects in fat absorption, especially of long-chain saturated fatty acids, but pancreatic triglyceride lipase did not appear to have a role in the fat malabsorption. Energy expenditure was higher in CCK-KO than wild-type mice, and CCK-KO mice had greater oxidation of carbohydrates while on the high-fat diet. Plasma leptin levels in the CCK-KO mice fed the high-fat diet were markedly lower than in wild-type mice, although levels of insulin, gastric-inhibitory polypeptide, and glucagon-like peptide-1 were normal.
Conclusions: CCK is involved in regulating the metabolic rate and is important for lipid absorption and control of body weight in mice placed on a high-fat diet.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No conflicts of interest exist for any of the authors listed above.
Figures

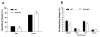
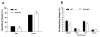


References
-
- Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. - PubMed
-
- Walsh JH. Gastrointesinal hormones. In: Johnson LR, Christensen J, Jackson MJ, Jacobson ED, Walsh JH, editors. Physiology of the gastrointesinal tract. second. New York: Raven Press; 1986. pp. 181–253.
-
- Muller K, Hsiao S. Specificity of cholecystokinin satiety effect: reduction of food but not water itnake. Pharmacol Biochem Behav. 1977;6:643–646. - PubMed
-
- Kraly FS, Carty WJ, Resnick S, Smith GP. Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J Comp Physiol Psychol. 1978;92:697–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

