Chemokines, neuronal-glial interactions, and central processing of neuropathic pain
- PMID: 20117131
- PMCID: PMC2839017
- DOI: 10.1016/j.pharmthera.2010.01.002
Chemokines, neuronal-glial interactions, and central processing of neuropathic pain
Abstract
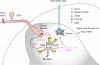
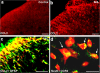
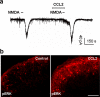
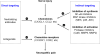
Millions of people worldwide suffer from neuropathic pain as a result of damage to or dysfunction of the nervous system under various disease conditions. Development of effective therapeutic strategies requires a better understanding of molecular and cellular mechanisms underlying the pathogenesis of neuropathic pain. It has been increasingly recognized that spinal cord glial cells such as microglia and astrocytes play a critical role in the induction and maintenance of neuropathic pain by releasing powerful neuromodulators such as proinflammatory cytokines and chemokines. Recent evidence reveals chemokines as new players in pain control. In this article, we review evidence for chemokine modulation of pain via neuronal-glial interactions by focusing on the central role of two chemokines, CX3CL1 (fractalkine) and CCL2 (MCP-1), because they differentially regulate neuronal-glial interactions. Release of CX3CL1 from neurons is ideal to mediate neuronal-to-microglial signaling, since the sole receptor of this chemokine, CX3CR1, is expressed in spinal microglia and activation of the receptor leads to phosphorylation of p38 MAP kinase in microglia. Although CCL2 was implicated in neuronal-to-microglial signaling, a recent study shows a novel role of CCL2 in astroglial-to-neuronal signaling after nerve injury. In particular, CCL2 rapidly induces central sensitization by increasing the activity of NMDA receptors in dorsal horn neurons. Insights into the role of chemokines in neuronal-glial interactions after nerve injury will identify new targets for therapeutic intervention of neuropathic pain.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain.J Pain. 2005 Jul;6(7):434-8. doi: 10.1016/j.jpain.2005.02.001. J Pain. 2005. PMID: 15993821
-
Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain.Cell Mol Life Sci. 2017 Sep;74(18):3275-3291. doi: 10.1007/s00018-017-2513-1. Epub 2017 Apr 7. Cell Mol Life Sci. 2017. PMID: 28389721 Free PMC article. Review.
-
Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions.Eur J Neurosci. 2004 Sep;20(5):1150-60. doi: 10.1111/j.1460-9568.2004.03593.x. Eur J Neurosci. 2004. PMID: 15341587
-
Role of the immune system in neuropathic pain.Scand J Pain. 2019 Dec 18;20(1):33-37. doi: 10.1515/sjpain-2019-0138. Scand J Pain. 2019. PMID: 31730538 Review.
-
Chemokines CCL2 and CCL7, but not CCL12, play a significant role in the development of pain-related behavior and opioid-induced analgesia.Cytokine. 2019 Jul;119:202-213. doi: 10.1016/j.cyto.2019.03.007. Epub 2019 Apr 16. Cytokine. 2019. PMID: 31003094
Cited by
-
Emerging role of Toll-like receptors in the control of pain and itch.Neurosci Bull. 2012 Apr;28(2):131-44. doi: 10.1007/s12264-012-1219-5. Neurosci Bull. 2012. PMID: 22466124 Free PMC article. Review.
-
Neuroimmune Mechanisms in Signaling of Pain During Acute Kidney Injury (AKI).Front Med (Lausanne). 2020 Aug 7;7:424. doi: 10.3389/fmed.2020.00424. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32850914 Free PMC article. Review.
-
Transient Receptor Potential Channels in Microglia: Roles in Physiology and Disease.Neurotox Res. 2016 Oct;30(3):467-78. doi: 10.1007/s12640-016-9632-6. Epub 2016 Jun 3. Neurotox Res. 2016. PMID: 27260222 Review.
-
Neuroinflammation in the peripheral nerve: Cause, modulator, or bystander in peripheral neuropathies?Glia. 2016 Apr;64(4):475-86. doi: 10.1002/glia.22899. Epub 2015 Aug 6. Glia. 2016. PMID: 26250643 Free PMC article. Review.
-
Interferon-gamma potentiates NMDA receptor signaling in spinal dorsal horn neurons via microglia-neuron interaction.Mol Pain. 2016 Apr 18;12:1744806916644927. doi: 10.1177/1744806916644927. Print 2016. Mol Pain. 2016. PMID: 27094552 Free PMC article.
References
-
- Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. - PubMed
-
- Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. - PubMed
-
- Arruda JL, Colburn RW, Rickman AJ, Rutkowski MD, DeLeo JA. Increase of interleukin-6 mRNA in the spinal cord following peripheral nerve injury in the rat: potential role of IL-6 in neuropathic pain. Brain Res Mol Brain Res. 1998;62:228–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

