Injectable intratumoral depot of thermally responsive polypeptide-radionuclide conjugates delays tumor progression in a mouse model
- PMID: 20117157
- PMCID: PMC2862899
- DOI: 10.1016/j.jconrel.2010.01.032
Injectable intratumoral depot of thermally responsive polypeptide-radionuclide conjugates delays tumor progression in a mouse model
Abstract
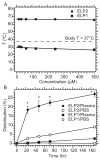
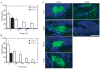
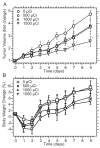
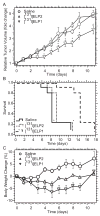
This study evaluated a biodegradable drug delivery system for local cancer radiotherapy consisting of a thermally sensitive elastin-like polypeptide (ELP) conjugated to a therapeutic radionuclide. Two ELPs (49 kDa) were synthesized using genetic engineering to test the hypothesis that injectable biopolymeric depots can retain radionuclides locally and reduce the growth of tumors. A thermally sensitive polypeptide, ELP(1), was designed to spontaneously undergo a soluble-insoluble phase transition (forming viscous microparticles) between room temperature and body temperature upon intratumoral injection, while ELP(2) was designed to remain soluble upon injection and to serve as a negative control for the effect of aggregate assembly. After intratumoral administration of radionuclide conjugates of ELPs into implanted tumor xenografts in nude mice, their retention within the tumor, spatio-temporal distribution, and therapeutic effect were quantified. The residence time of the radionuclide-ELP(1) in the tumor was significantly longer than the thermally insensitive ELP(2) conjugate. In addition, the thermal transition of ELP(1) significantly protected the conjugated radionuclide from dehalogenation, whereas the conjugated radionuclide on ELP(2) was quickly eliminated from the tumor and cleaved from the biopolymer. These attributes of the thermally sensitive ELP(1) depot improved the antitumor efficacy of iodine-131 compared to the soluble ELP(2) control. This novel injectable and biodegradable depot has the potential to control advanced-stage cancers by reducing the bulk of inoperable tumors, enabling surgical removal of de-bulked tumors, and preserving healthy tissues.
Published by Elsevier B.V.
Conflict of interest statement
A.C. has a financial interest in Phase Biopharmaceuticals, which has licensed the rights to the local delivery technology described herein from Duke University.
Figures
References
-
- Celikoglu F, Celikoglu SI, York AM, Goldberg EP. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer. 2006;51(2):225–236. - PubMed
-
- Nakase Y, Hagiwara A, Kin S, Fukuda K, Ito T, Takagi T, Fujiyama J, Sakakura C, Otsuji E, Yamagishi H. Intratumoral administration of methotrexate bound to activated carbon particles: antitumor effectiveness against human colon carcinoma xenografts and acute toxicity in mice. J Pharmacol Exp Ther. 2004;311(1):382–387. - PubMed
-
- van Herpen CM, van der Laak JA, de Vries IJ, van Krieken JH, de Wilde PC, Balvers MG, Adema GJ, De Mulder PH. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin Cancer Res. 2005;11(5):1899–1909. - PubMed
-
- Li YC, Xu WY, Tan TZ, He S. 131I-recombinant human EGF has antitumor effects against MCF-7 human breast cancer xenografts with low levels of EGFR. Nucl Med Biol. 2004;31(4):435–440. - PubMed
-
- Rosemurgy A, Luzardo G, Cooper J, Bowers C, Zervos E, Bloomston M, Al-Saadi S, Carroll R, Chheda H, Carey L, Goldin S, Grundy S, Kudryk B, Zwiebel B, Black T, Briggs J, Chervenick P. 32P as an adjunct to standard therapy for locally advanced unresectable pancreatic cancer: a randomized trial. J Gastrointest Surg. 2008;12(4):682–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

