Live-cell analysis of kinetochore-microtubule interaction in budding yeast
- PMID: 20117214
- PMCID: PMC2954359
- DOI: 10.1016/j.ymeth.2010.01.017
Live-cell analysis of kinetochore-microtubule interaction in budding yeast
Abstract
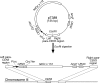
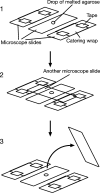
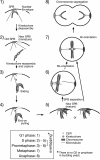
Kinetochore capture and transport by spindle microtubules plays a crucial role in high-fidelity chromosome segregation, although its detailed mechanism has remained elusive. It has been difficult to observe individual kinetochore-microtubule interactions because multiple kinetochores are captured by microtubules during a short period within a small space. We have developed a method to visualize individual kinetochore-microtubule interactions in Saccharomyces cerevisiae, by isolating one of the kinetochores from others through regulation of the activity of a centromere. We detail this technique, which we call 'centromere reactivation system', for dissection of the process of kinetochore capture and transport on mitotic spindle. Kinetochores are initially captured by the side of microtubules extending from a spindle pole, and subsequently transported poleward along them, which is an evolutionarily conserved process from yeast to vertebrate cells. Our system, in combination with amenable yeast genetics, has proved useful to elucidate the molecular mechanisms of kinetochore-microtubule interactions. We discuss practical considerations for applying our system to live cell imaging using fluorescence microscopy.
Figures
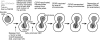

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

