HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters
- PMID: 20117238
- PMCID: PMC2838954
- DOI: 10.1016/j.bbalip.2010.01.007
HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters
Abstract
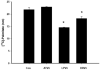
Infection with human immunodeficiency virus (HIV) and treatment with HIV-protease inhibitor (PI)-based highly active antiretroviral therapies (HAART) is associated with dysregulated fatty acid and lipid metabolism. Enhanced lipolysis, increased circulating fatty acid levels, and hepatic and intramuscular lipid accumulation appear to contribute to insulin resistance in HIV-infected people treated with PI-based HAART. However, it is unclear whether currently prescribed HIV-PIs directly alter skeletal muscle fatty acid transport, oxidation, and storage. We find that ritonavir (r, 5micromol/l) plus 20micromol/l of atazanavir (ATV), lopinavir (LPV), or darunavir (DRV) reduce palmitate oxidation(16-21%) in differentiated C2C12 myotubes. Palmitate oxidation was increased following exposure to high fatty acid media but this effect was blunted when myotubes were pre-exposed to the HIV-PIs. However, LPV/r and DRV/r, but not ATV/r suppressed palmitate uptake into myotubes. We found no effect of the HIV-PIs on FATP1, FATP4, or FABPpm but both CD36/FAT and carnitine palmitoyltransferase 1 (CPT1) were reduced by all three regimens though ATV/r caused only a small decrease in CPT1, relative to LPV/r or DRV/r. In contrast, sterol regulatory element binding protein-1 was increased by all 3 HIV-PIs. These findings suggest that HIV-PIs suppress fatty acid oxidation in murine skeletal muscle cells and that this may be related to decreases in cytosolic- and mitochondrial-associated fatty acid transporters. HIV-PIs may also directly impair fatty acid handling and partitioning in skeletal muscle, and this may contribute to the cluster of metabolic complications that occur in people living with HIV.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures







Similar articles
-
Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells.J Lipid Res. 2009 Sep;50(9):1789-99. doi: 10.1194/jlr.M800535-JLR200. Epub 2009 May 9. J Lipid Res. 2009. PMID: 19429947 Free PMC article.
-
Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle.J Biol Chem. 2009 Jun 12;284(24):16522-16530. doi: 10.1074/jbc.M109.004788. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380575 Free PMC article.
-
Impact of darunavir, atazanavir and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin.Antivir Ther. 2014;19(8):773-82. doi: 10.3851/IMP2752. Epub 2014 Feb 17. Antivir Ther. 2014. PMID: 24535489
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
Cited by
-
Body composition and metabolic changes in HIV-infected patients.J Infect Dis. 2012 Jun;205 Suppl 3(Suppl 3):S383-90. doi: 10.1093/infdis/jis205. J Infect Dis. 2012. PMID: 22577212 Free PMC article. Review.
-
Skeletal muscle cellular metabolism in older HIV-infected men.Physiol Rep. 2016 May;4(9):e12794. doi: 10.14814/phy2.12794. Physiol Rep. 2016. PMID: 27166139 Free PMC article.
-
Changes in plasma lipidome following initiation of antiretroviral therapy.PLoS One. 2018 Aug 29;13(8):e0202944. doi: 10.1371/journal.pone.0202944. eCollection 2018. PLoS One. 2018. PMID: 30157268 Free PMC article.
-
Plasma Lipidomic Profiles in cART-Treated Adolescents with Perinatally Acquired HIV Compared to Matched Controls.Viruses. 2024 Apr 9;16(4):580. doi: 10.3390/v16040580. Viruses. 2024. PMID: 38675922 Free PMC article.
-
An obese body mass increases the adverse effects of HIV/AIDS on balance and gait.Phys Ther. 2011 Jul;91(7):1063-71. doi: 10.2522/ptj.20100292. Epub 2011 Apr 28. Phys Ther. 2011. PMID: 21527386 Free PMC article.
References
-
- Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK069455/DK/NIDDK NIH HHS/United States
- R01 DK059531/DK/NIDDK NIH HHS/United States
- R01-DK69455/DK/NIDDK NIH HHS/United States
- T32-DK007296-27/DK/NIDDK NIH HHS/United States
- DK049393/DK/NIDDK NIH HHS/United States
- P30-DK-056341/DK/NIDDK NIH HHS/United States
- DK074345/DK/NIDDK NIH HHS/United States
- DK059531/DK/NIDDK NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R56 DK049393/DK/NIDDK NIH HHS/United States
- R01 DK049393/DK/NIDDK NIH HHS/United States
- R21 DK074345/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P60-DK020579/DK/NIDDK NIH HHS/United States
- R21 AT003083/AT/NCCIH NIH HHS/United States
- T32 DK007296/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

