Understanding protein non-folding
- PMID: 20117254
- PMCID: PMC2882790
- DOI: 10.1016/j.bbapap.2010.01.017
Understanding protein non-folding
Abstract
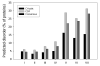
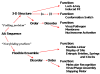
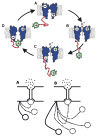
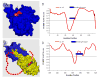
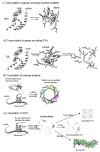
This review describes the family of intrinsically disordered proteins, members of which fail to form rigid 3-D structures under physiological conditions, either along their entire lengths or only in localized regions. Instead, these intriguing proteins/regions exist as dynamic ensembles within which atom positions and backbone Ramachandran angles exhibit extreme temporal fluctuations without specific equilibrium values. Many of these intrinsically disordered proteins are known to carry out important biological functions which, in fact, depend on the absence of a specific 3-D structure. The existence of such proteins does not fit the prevailing structure-function paradigm, which states that a unique 3-D structure is a prerequisite to function. Thus, the protein structure-function paradigm has to be expanded to include intrinsically disordered proteins and alternative relationships among protein sequence, structure, and function. This shift in the paradigm represents a major breakthrough for biochemistry, biophysics and molecular biology, as it opens new levels of understanding with regard to the complex life of proteins. This review will try to answer the following questions: how were intrinsically disordered proteins discovered? Why don't these proteins fold? What is so special about intrinsic disorder? What are the functional advantages of disordered proteins/regions? What is the functional repertoire of these proteins? What are the relationships between intrinsically disordered proteins and human diseases?
Copyright 2010 Elsevier B.V. All rights reserved.
Figures


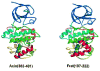

References
-
- Fischer E. Einfluss der configuration auf die wirkung der enzyme. Ber Dt Chem Ges. 1894;27:2985–2993.
-
- Lemieux UR, Spohr U. How Emil Fischer was led to the lock and key concept for enzyme specificity. Adv Carbohydrate Chem Biochem. 1994;50:1–20. - PubMed
-
- Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965;206:757–761. - PubMed
-
- Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 1958;181:662–666. - PubMed
-
- Kendrew JC, Dickerson RE, Stranberg BE, H RJ, Davies DR, Phillips DC, Shore VC. Structure of myoglobin: a three-dimensional Fourier synthesis at 2 Å resolution. Nature. 1960;185:422–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

