Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial
- PMID: 20118174
- PMCID: PMC2848325
- DOI: 10.1093/eurheartj/ehp604
Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial
Abstract
Aims: Thiazolidinediones are insulin sensitizers, and are associated with fluid retention and increased risk of heart failure (HF) in people with type 2 diabetes. We assessed fatal and non-fatal HF events and their outcome, and identified HF predictors in the RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycaemia in Diabetes) trial population.
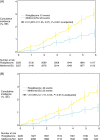
Methods and results: In a multicentre, open-label study, we randomized 4447 people with type 2 diabetes on metformin or sulfonylurea monotherapy with a mean HbA(1c) of 7.9% to add-on rosiglitazone (n = 2220) or to a combination of metformin and sulfonylurea (n = 2227) and followed them over 5.5 years on average. Heart failure hospitalizations and deaths were adjudicated by a Clinical Endpoint Committee using pre-specified criteria. Independent predictors of HF events were identified out of a group of 30 pre-specified clinical, demographic, and biological variables. In the rosiglitazone group, the risk of HF death or hospitalization was doubled: HR = 2.10 (95% CI, 1.35-3.27): the excess HF event rate was 2.6 (1.1-4.1) per 1000 person-years. An excess in HF deaths was observed (10 vs. two), including four HF deaths as first HF events. By contrast, there was no increase in cardiovascular mortality or hospitalization (HR = 0.99, 95% CI, 0.85-1.16) or in cardiovascular deaths (60 vs. 71). Independent predictors of HF were rosiglitazone assignment, age, urinary albumin : creatinine ratio, body mass index, and systolic blood pressure at baseline. A history of previous cardiovascular disease was not predictive of HF. Duration of HF hospitalization and rate of HF re-hospitalization were similar in the two groups.
Conclusion: These findings confirm the increased risk of HF events in people treated with rosiglitazone and support the recommendation that this agent should not continue to be used in people developing symptomatic HF while using the medication. Close follow-up for the risk of HF should be offered to elderly people, people with markedly increased body mass index, people with microalbuminuria/proteinuria, and people with increased systolic blood pressure.
Figures
Comment in
-
The rise and fall of rosiglitazone.Eur Heart J. 2010 Apr;31(7):773-6. doi: 10.1093/eurheartj/ehq016. Epub 2010 Feb 12. Eur Heart J. 2010. PMID: 20154334 No abstract available.
References
-
- Delea TE, Edelsberg JS, Hagiwara M, Oster G, Philips LS. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2003;26:2983–2989. - PubMed
-
- Kermani A, Garg A. Thiazolidinedione-associated congestive heart failure and pulmonary edema. Mayo Clin Proc. 2003;78:1088–1091. - PubMed
-
- Wilson TWH, Francis GS, Hoogwerf J, Young JB. Fluid retention after initiation of thiazolidinedione therapy in diabetic patients with established chronic heart failure. J Am Coll Cardiol. 2003;41:1394–1398. - PubMed
-
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–2948. - PubMed
-
- Dormandy JA, Charbonnel B, Eckland EJA, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Joranyi L, Laakso M, Makon M, Norkus A, Pirags V, Podar R, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes: a randomized trial of pioglitazone. The PROactive Study (PROspective pioglitAzone Clinical Trial In macro Vascular Events) Lancet. 2005;366:1279–1289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous