Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss
- PMID: 20118184
- PMCID: PMC2936803
- DOI: 10.1093/cercor/bhp317
Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss
Abstract
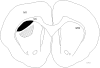
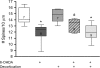
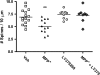
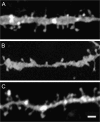
Striatal medium spiny neurons (MSNs) receive glutamatergic afferents from the cerebral cortex and dopaminergic inputs from the substantia nigra (SN). Striatal dopamine loss decreases the number of MSN dendritic spines. This loss of spines has been suggested to reflect the removal of tonic dopamine inhibitory control over corticostriatal glutamatergic drive, with increased glutamate release culminating in MSN spine loss. We tested this hypothesis in two ways. We first determined in vivo if decortication reverses or prevents dopamine depletion-induced spine loss by placing motor cortex lesions 4 weeks after, or at the time of, 6-hydroxydopamine lesions of the SN. Animals were sacrificed 4 weeks after cortical lesions. Motor cortex lesions significantly reversed the loss of MSN spines elicited by dopamine denervation; a similar effect was observed in the prevention experiment. We then determined if modulating glutamate release in organotypic cocultures prevented spine loss. Treatment of the cultures with the mGluR2/3 agonist LY379268 to suppress corticostriatal glutamate release completely blocked spine loss in dopamine-denervated cultures. These studies provide the first evidence to show that MSN spine loss associated with parkinsonism can be reversed and point to suppression of corticostriatal glutamate release as a means of slowing progression in Parkinson's disease.
Figures



Similar articles
-
Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons.Neuroscience. 2007 Oct 26;149(2):457-64. doi: 10.1016/j.neuroscience.2007.06.044. Epub 2007 Jul 17. Neuroscience. 2007. PMID: 17888581 Free PMC article.
-
Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism.Parkinsonism Relat Disord. 2007;13 Suppl 3(Suppl 3):S251-8. doi: 10.1016/S1353-8020(08)70012-9. Parkinsonism Relat Disord. 2007. PMID: 18267246 Free PMC article. Review.
-
Remodeling of the dendritic structure of the striatal medium spiny neurons accompanies behavioral recovery in a mouse model of Parkinson's disease.Neurosci Lett. 2013 Dec 17;557 Pt B:95-100. doi: 10.1016/j.neulet.2013.10.049. Epub 2013 Oct 28. Neurosci Lett. 2013. PMID: 24176882
-
Striatal spine plasticity in Parkinson's disease: pathological or not?Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3(Suppl 3):S156-61. doi: 10.1016/S1353-8020(09)70805-3. Parkinsonism Relat Disord. 2009. PMID: 20082980 Free PMC article.
-
Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review.
Cited by
-
Developmental Changes in Dendritic Spine Morphology in the Striatum and Their Alteration in an A53T α-Synuclein Transgenic Mouse Model of Parkinson's Disease.eNeuro. 2020 Aug 27;7(4):ENEURO.0072-20.2020. doi: 10.1523/ENEURO.0072-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32817196 Free PMC article.
-
Partial decortication ameliorates dopamine depletion‑induced striatal neuron lesions in rats.Int J Mol Med. 2019 Oct;44(4):1414-1424. doi: 10.3892/ijmm.2019.4288. Epub 2019 Jul 25. Int J Mol Med. 2019. PMID: 31364729 Free PMC article.
-
Practical Strategies and Concepts in GPCR Allosteric Modulator Discovery: Recent Advances with Metabotropic Glutamate Receptors.Chem Rev. 2016 Jun 8;116(11):6707-41. doi: 10.1021/acs.chemrev.5b00656. Epub 2016 Feb 16. Chem Rev. 2016. PMID: 26882314 Free PMC article. Review.
-
Subcellular location of PKA controls striatal plasticity: stochastic simulations in spiny dendrites.PLoS Comput Biol. 2012 Feb;8(2):e1002383. doi: 10.1371/journal.pcbi.1002383. Epub 2012 Feb 9. PLoS Comput Biol. 2012. PMID: 22346744 Free PMC article.
-
Morphological changes in perisynaptic astrocytes induced by dopamine neuronal degeneration in the striatum of rats.Heliyon. 2024 Mar 5;10(6):e27637. doi: 10.1016/j.heliyon.2024.e27637. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38510046 Free PMC article.
References
-
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol. 2006;499:33–48. - PubMed
-
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

