Pharmacokinetics of metformin during pregnancy
- PMID: 20118196
- PMCID: PMC2872944
- DOI: 10.1124/dmd.109.031245
Pharmacokinetics of metformin during pregnancy
Abstract
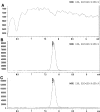
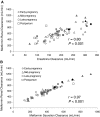
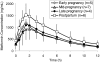
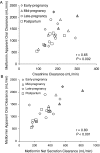
Our objective was to evaluate the pharmacokinetics of metformin during pregnancy. Serial blood and urine samples were collected over one steady-state dosing interval in women treated with metformin during early to late pregnancy (n = 35) and postpartum (n = 16). Maternal and umbilical cord blood samples were obtained at delivery from 12 women. Metformin concentrations were also determined in breast milk samples obtained over one dosing interval in 6 women. Metformin renal clearance increased significantly in mid (723 +/- 243 ml/min, P < 0.01) and late pregnancy (625 +/- 130 ml/min, P < 0.01) compared with postpartum (477 +/- 132 ml/min). These changes reflected significant increases in creatinine clearance (240 +/- 70 ml/min, P < 0.01 and 207 +/- 56 ml/min, P < 0.05 versus 165 +/- 44 ml/min) and in metformin net secretion clearance (480 +/- 190 ml/min, P < 0.01 and 419 +/- 78 ml/min, P < 0.01 versus 313 +/- 98 ml/min) in mid and late pregnancy versus postpartum, respectively. Metformin concentrations at the time of delivery in umbilical cord plasma ranged between nondetectable (<5 ng/ml) and 1263 ng/ml. The daily infant intake of metformin through breast milk was 0.13 to 0.28 mg, and the relative infant dose was <0.5% of the mother's weight-adjusted dose. Our results indicate that metformin pharmacokinetics are affected by pregnancy-related changes in renal filtration and net tubular transport and can be roughly estimated by the use of creatinine clearance. At the time of delivery, the fetus is exposed to metformin concentrations from negligible to as high as maternal concentrations. In contrast, infant exposure to metformin through the breast milk is low.
Figures


References
-
- Alnouti Y, Petrick JS, Klaassen CD. (2006) Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos 34:477–482 - PubMed
-
- American Academy of Pediatrics Work Group on Breastfeeding (1997) Breastfeeding and the use of human milk. Pediatrics 100:1035–1039 - PubMed
-
- Andrew MA, Easterling TR, Carr DB, Shen D, Buchanan ML, Rutherford T, Bennett R, Vicini P, Hebert MF. (2007) Amoxicillin pharmacokinetics in pregnant women: modeling and simulations of dosage strategies. Clin Pharmacol Ther 81:547–556 - PubMed
-
- Briggs GG, Ambrose PJ, Nageotte MP, Padilla G, Wan S. (2005) Excretion of metformin into breast milk and the effect on nursing infants. Obstet Gynecol 105:1437–1441 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1-RR025014/RR/NCRR NIH HHS/United States
- U10-HD047892/HD/NICHD NIH HHS/United States
- T32 RR023256/RR/NCRR NIH HHS/United States
- RR023256/RR/NCRR NIH HHS/United States
- M01-RR00037/RR/NCRR NIH HHS/United States
- M01 RR000037/RR/NCRR NIH HHS/United States
- U10 HD047890/HD/NICHD NIH HHS/United States
- U10-HD047890/HD/NICHD NIH HHS/United States
- U10 HD047891/HD/NICHD NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- M01 RR023942/RR/NCRR NIH HHS/United States
- U10-HD047891/HD/NICHD NIH HHS/United States
- M01-RR023942/RR/NCRR NIH HHS/United States
- U10 HD047892/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

