A pharmacologically active monoclonal antibody against the human melanocortin-4 receptor: effectiveness after peripheral and central administration
- PMID: 20118207
- PMCID: PMC3202465
- DOI: 10.1124/jpet.109.163279
A pharmacologically active monoclonal antibody against the human melanocortin-4 receptor: effectiveness after peripheral and central administration
Abstract
The hypothalamic melanocortin-4 receptor (MC4R) is a constituent of an important pathway regulating food intake and energy expenditure. We produced a monoclonal antibody (mAb) directed against the N-terminal domain of the MC4R and evaluated its potential as a possible therapeutic agent. This mAb (1E8a) showed specific binding to the MC4R in human embryonic kidney 293 cells expressing the human MC4R and blocked the activity of the MC4R under basal conditions and after stimulation with alpha-melanocyte-stimulating hormone (alpha-MSH). The inverse agonist action of Agouti-related protein was significantly enhanced in the presence of mAb 1E8a. After a single intracerebroventricular injection into the third ventricle, mAb 1E8a (1 microg) increased 24-h food intake in rats. After 7 days of continuous intracerebroventricular administration, mAb 1E8a increased food intake, body weight, and fat pad weight and induced hyperglycemia. Because the complete mAb was ineffective after intravenous injection, we produced single-chain variable fragments (scFvs) derived from mAb 1E8a. In pharmacokinetic studies it was demonstrated that these scFvs crossed the blood-brain barrier and reached the hypothalamus. Consequently, the scFv 1E8a increased significantly food intake and body weight in rats after intravenous administration (300 mug/kg). The pharmacological profile of mAb 1E8a and the fact that its scFv was active after peripheral administration suggest that derivatives of anti-MC4R mAbs may be useful in the treatment of patients with anorexia or cachexia.
Figures

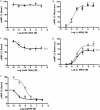
 ). The presence of mAb 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curves obtained with purified mAb 1E8a. The concentration-dependent decrease in the intracellular cAMP content suggests an inverse agonist effect of mAb 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 100 nM mAb 1E8a (♦) or PBS (
). The presence of mAb 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curves obtained with purified mAb 1E8a. The concentration-dependent decrease in the intracellular cAMP content suggests an inverse agonist effect of mAb 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 100 nM mAb 1E8a (♦) or PBS (
 ). The reduced maximum efficacy of α-MSH in the presence of mAb 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. e, concentration-response curves obtained with AgRP in the presence of 100 nM mAb 1E8a (♦) or PBS (
). The reduced maximum efficacy of α-MSH in the presence of mAb 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. e, concentration-response curves obtained with AgRP in the presence of 100 nM mAb 1E8a (♦) or PBS (
 ). The leftward shift of the AgRP response curve in the presence of mAb 1E8a suggests that this mAb acts in synergy with AgRP. Data are presented as means ± S.D. calculated from three independent experiments. ***, p < 0.001, F test.
). The leftward shift of the AgRP response curve in the presence of mAb 1E8a suggests that this mAb acts in synergy with AgRP. Data are presented as means ± S.D. calculated from three independent experiments. ***, p < 0.001, F test.




 ) or PBS (
) or PBS (
 ). The presence of the scFv 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curve obtained with purified scFv 1E8a. The concentration-dependent decrease in cAMP formation suggests an inverse agonist effect of the scFv 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 10 nM scFv 1E8a (⋄), 100 nM scFv 1E8a (♦), or PBS (▪). The reduced maximum efficacy of α-MSH in the presence of scFv 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. **, p < 0.01; ***, p < 0.001, F test.
). The presence of the scFv 2G2 had no effect on the concentration-response curve of α-MSH. c, concentration-response curve obtained with purified scFv 1E8a. The concentration-dependent decrease in cAMP formation suggests an inverse agonist effect of the scFv 1E8a. d, concentration-response curves obtained with α-MSH in the presence or absence of 10 nM scFv 1E8a (⋄), 100 nM scFv 1E8a (♦), or PBS (▪). The reduced maximum efficacy of α-MSH in the presence of scFv 1E8a suggests that this mAb acts as a noncompetitive antagonist. Data are presented as means ± S.D. calculated from three independent experiments. **, p < 0.01; ***, p < 0.001, F test.

References
-
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. - PubMed
-
- Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide1–27 and pituitary adenylate cyclase activating polypeptide1–38 across the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:690–696. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

