Temporal relationship between primary and motile ciliogenesis in airway epithelial cells
- PMID: 20118219
- PMCID: PMC2993092
- DOI: 10.1165/rcmb.2009-0328OC
Temporal relationship between primary and motile ciliogenesis in airway epithelial cells
Abstract
Cilia are traditionally classified as motile or primary. Motile cilia are restricted to specific populations of well-differentiated epithelial cells, including those in the airway, brain ventricles, and oviducts. Primary cilia are nonmotile, solitary structures that are present in many cell types, and often have sensory functions such as in the retina and renal tubules. Primary cilia were also implicated in the regulation of fundamental processes in development. Rare depictions of primary cilia in embryonic airways led us to hypothesize that primary cilia in airway cells are temporally related to motile ciliogenesis. We identified primary cilia in undifferentiated, cultured airway epithelial cells from mice and humans and in developing lungs. The solitary cilia in the airways express proteins considered unique to primary cilia, including polycystin-1 and polycystin-2. A temporal analysis of airway epithelial cell differentiation showed that cells with primary cilia acquire markers of motile ciliogenesis, suggesting that motile ciliated cells originate from primary ciliated cells. Whereas motile ciliogenesis requires Foxj1, primary ciliogenesis does not, and the expression of Foxj1 was associated with a loss of primary cilia, just before the appearance of motile cilia. Primary cilia were not found in well-differentiated airway epithelial cells. However, after injury, they appear in the luminal layer of epithelium and in basal cells. The transient nature of primary cilia, together with the temporal and spatial patterns of expression in the development and repair of airway epithelium, suggests a critical role of primary cilia in determining outcomes during airway epithelial cell differentiation.
Figures

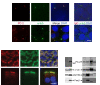


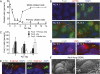
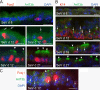

Similar articles
-
Identification of FOXJ1 effectors during ciliogenesis in the foetal respiratory epithelium and embryonic left-right organiser of the mouse.Dev Biol. 2017 Mar 15;423(2):170-188. doi: 10.1016/j.ydbio.2016.11.019. Epub 2016 Dec 1. Dev Biol. 2017. PMID: 27914912
-
Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2004 Apr;286(4):L650-7. doi: 10.1152/ajplung.00170.2003. Epub 2003 Jun 20. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 12818891
-
Motile cilia harbor serum response factor as a mechanism of environment sensing and injury response in the airway.Am J Physiol Lung Cell Mol Physiol. 2014 May 1;306(9):L829-39. doi: 10.1152/ajplung.00364.2013. Epub 2014 Mar 7. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24610937 Free PMC article.
-
Airway ciliated cells in adult lung homeostasis and COPD.Eur Respir Rev. 2023 Dec 6;32(170):230106. doi: 10.1183/16000617.0106-2023. Print 2023 Dec 31. Eur Respir Rev. 2023. PMID: 38056888 Free PMC article. Review.
-
Sensory functions of motile cilia and implication for bronchiectasis.Front Biosci (Schol Ed). 2012 Jan 1;4(3):1088-98. doi: 10.2741/s320. Front Biosci (Schol Ed). 2012. PMID: 22202111 Free PMC article. Review.
Cited by
-
Formation and function of multiciliated cells.J Cell Biol. 2024 Jan 1;223(1):e202307150. doi: 10.1083/jcb.202307150. Epub 2023 Nov 30. J Cell Biol. 2024. PMID: 38032388 Free PMC article. Review.
-
Attenuated Amiloride-Sensitive Current and Augmented Calcium-Activated Chloride Current in Marsh Rice Rat (Oryzomys palustris) Airways.iScience. 2019 Sep 27;19:737-748. doi: 10.1016/j.isci.2019.08.011. Epub 2019 Aug 8. iScience. 2019. PMID: 31491720 Free PMC article.
-
Long-range migration of centrioles to the apical surface of the olfactory epithelium.Elife. 2022 Apr 14;11:e74399. doi: 10.7554/eLife.74399. Elife. 2022. PMID: 35420544 Free PMC article.
-
Primary Cilium-Mediated Retinal Pigment Epithelium Maturation Is Disrupted in Ciliopathy Patient Cells.Cell Rep. 2018 Jan 2;22(1):189-205. doi: 10.1016/j.celrep.2017.12.038. Cell Rep. 2018. PMID: 29298421 Free PMC article.
-
A microfluidic device to apply shear stresses to polarizing ciliated airway epithelium using air flow.Biomicrofluidics. 2014 Nov 14;8(6):064104. doi: 10.1063/1.4901930. eCollection 2014 Nov. Biomicrofluidics. 2014. PMID: 25553181 Free PMC article.
References
-
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 2006;7:125–148. - PubMed
-
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 2007;8:880–893. - PubMed
-
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 2003;15:105–110. - PubMed
-
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol 2005;289:F1159–F1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

