Micromechanics of alveolar edema
- PMID: 20118224
- PMCID: PMC3028256
- DOI: 10.1165/rcmb.2009-0005OC
Micromechanics of alveolar edema
Abstract
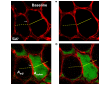
The decrease of lung compliance in pulmonary edema underlies ventilator-induced lung injury. However, the cause of the decrease in compliance is unknown. We tested the hypothesis that in pulmonary edema, the mechanical effects of liquid-filled alveoli increase tissue stress in adjacent air-filled alveoli. By micropuncture of isolated, perfused rat lungs, we established a single-alveolus model of pulmonary edema that we imaged using confocal microscopy. In this model, we viewed a liquid-filled alveolus together with its air-filled neighbor at different transpulmonary pressures, both before and after liquid-filling. Instilling liquid in an alveolus caused alveolar shrinkage. As a result, the interalveolar septum was stretched, causing the neighboring air-filled alveolus to bulge. Thus, the air-filled alveolus was overexpanded by virtue of its adjacency to a liquid-filled alveolus. Confocal microscopy at different depths of the liquid-filled alveolus revealed a meniscus. Lung inflation to near-total lung capacity (TLC) demonstrated decreased compliance of the air-filled but not liquid-filled alveolus. However, at near TLC, the air-filled alveolus was larger than it was in the pre-edematous control tissue. In pulmonary edema, liquid-filled alveoli induce mechanical stress on air-filled alveoli, reducing the compliance of air-filled alveoli, and hence overall lung compliance. Because of increased mechanical stress, air-filled alveoli may be susceptible to overdistension injury during mechanical ventilation of the edematous lung.
Figures



References
-
- Staub NC, Nagano H, Pearce ML. Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J Appl Physiol 1967;22:227–240. - PubMed
-
- Bachofen H, Schurch S, Michel RP, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs. Morphology. Am Rev Respir Dis 1993;147:989–996. - PubMed
-
- Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol 2007;103:1037–1044. - PubMed
-
- Weibel ER, Gomez DM. Architecture of the human lung. Science 1962;137:577–585. - PubMed
-
- Von Neergaard K. Neue Auffassungen über einen Grundbegriff der Atemmechanik. die Retraktionskraft der Lunge, abhangig von der Oberflachenspannung in den Alveolen. Z Gesamte Exp Med 1929;66:373–394.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

