Last step in the conversion of trehalose to glycogen: a mycobacterial enzyme that transfers maltose from maltose 1-phosphate to glycogen
- PMID: 20118231
- PMCID: PMC2843229
- DOI: 10.1074/jbc.M109.033944
Last step in the conversion of trehalose to glycogen: a mycobacterial enzyme that transfers maltose from maltose 1-phosphate to glycogen
Abstract
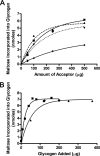
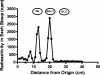
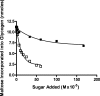
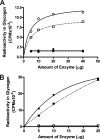
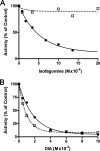
We show that Mycobacterium smegmatis has an enzyme catalyzing transfer of maltose from [(14)C]maltose 1-phosphate to glycogen. This enzyme was purified 90-fold from crude extracts and characterized. Maltose transfer required addition of an acceptor. Liver, oyster, or mycobacterial glycogens were the best acceptors, whereas amylopectin had good activity, but amylose was a poor acceptor. Maltosaccharides inhibited the transfer of maltose from [(14)C]maltose-1-P to glycogen because they were also acceptors of maltose, and they caused production of larger sized radioactive maltosaccharides. When maltotetraose was the acceptor, over 90% of the (14)C-labeled product was maltohexaose, and no radioactivity was in maltopentaose, demonstrating that maltose was transferred intact. Stoichiometry showed that 0.89 micromol of inorganic phosphate was produced for each micromole of maltose transferred to glycogen, and 56% of the added maltose-1-P was transferred to glycogen. This enzyme has been named alpha1,4-glucan:maltose-1-P maltosyltransferase (GMPMT). Transfer of maltose to glycogen was inhibited by micromolar amounts of inorganic phosphate or arsenate but was only slightly inhibited by millimolar concentrations of glucose-1-P, glucose-6-P, or inorganic pyrophosphate. GMPMT was compared with glycogen phosphorylase (GP). GMPMT catalyzed transfer of [(14)C]maltose-1-P, but not [(14)C]glucose-1-P, to glycogen, whereas GP transferred radioactivity from glucose-1-P but not maltose-1-P. GMPMT and GP were both inhibited by 1,4-dideoxy-1,4-imino-d-arabinitol, but only GP was inhibited by isofagomine. Because mycobacteria that contain trehalose synthase accumulate large amounts of glycogen when grown in high concentrations of trehalose, we propose that trehalose synthase, maltokinase, and GMPMT represent a new pathway of glycogen synthesis using trehalose as the source of glucose.
Figures


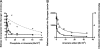
References
-
- Elbein A. D. (1974) Adv. Carbohydr. Chem. Biochem. 30, 227–256 - PubMed
-
- Nwaka S., Holzer H. (1998) Prog. Nucleic Acid Res. Mol. Biol. 58, 197–237 - PubMed
-
- Bell W., Klaassen P., Ohnacker M., Boller T., Herweijer M., Schoppink P., Van der Zee P., Wiemken A. (1992) Eur. J. Biochem. 209, 951–959 - PubMed
-
- Crowe J. H., Hoekstra F. A., Crowe L. M. (1992) Annu. Rev. Physiol. 54, 579–599 - PubMed
-
- Takayama K., Armstrong E. L. (1976) Biochemistry 15, 441–446 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

