Alternative route for glyoxylate consumption during growth on two-carbon compounds by Methylobacterium extorquens AM1
- PMID: 20118267
- PMCID: PMC2838054
- DOI: 10.1128/JB.01166-09
Alternative route for glyoxylate consumption during growth on two-carbon compounds by Methylobacterium extorquens AM1
Abstract
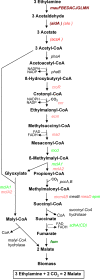
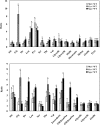
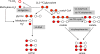
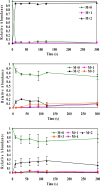
Methylobacterium extorquens AM1 is a facultative methylotroph capable of growth on both single-carbon and multicarbon compounds. Mutants defective in a pathway involved in converting acetyl-coenzyme A (CoA) to glyoxylate (the ethylmalonyl-CoA pathway) are unable to grow on both C(1) and C(2) compounds, showing that both modes of growth have this pathway in common. However, growth on C(2) compounds via the ethylmalonyl-CoA pathway should require glyoxylate consumption via malate synthase, but a mutant lacking malyl-CoA/beta-methylmalyl-CoA lyase activity (MclA1) that is assumed to be responsible for malate synthase activity still grows on C(2) compounds. Since glyoxylate is toxic to this bacterium, it seemed likely that a system is in place to keep it from accumulating. In this study, we have addressed this question and have shown by microarray analysis, mutant analysis, metabolite measurements, and (13)C-labeling experiments that M. extorquens AM1 contains an additional malyl-CoA/beta-methylmalyl-CoA lyase (MclA2) that appears to take part in glyoxylate metabolism during growth on C(2) compounds. In addition, an alternative pathway appears to be responsible for consuming part of the glyoxylate, converting it to glycine, methylene-H(4)F, and serine. Mutants lacking either pathway have a partial defect for growth on ethylamine, while mutants lacking both pathways are unable to grow appreciably on ethylamine. Our results suggest that the malate synthase reaction is a bottleneck for growth on C(2) compounds by this bacterium, which is partially alleviated by this alternative route for glyoxylate consumption. This strategy of multiple enzymes/pathways for the consumption of a toxic intermediate reflects the metabolic versatility of this facultative methylotroph and is a model for other metabolic networks involving high flux through toxic intermediates.
Figures


Similar articles
-
Replacing the Ethylmalonyl-CoA Pathway with the Glyoxylate Shunt Provides Metabolic Flexibility in the Central Carbon Metabolism of Methylobacterium extorquens AM1.ACS Synth Biol. 2018 Jan 19;7(1):86-97. doi: 10.1021/acssynbio.7b00229. Epub 2017 Dec 21. ACS Synth Biol. 2018. PMID: 29216425
-
Ethylmalonyl coenzyme A mutase operates as a metabolic control point in Methylobacterium extorquens AM1.J Bacteriol. 2015 Feb 15;197(4):727-35. doi: 10.1128/JB.02478-14. Epub 2014 Dec 1. J Bacteriol. 2015. PMID: 25448820 Free PMC article.
-
Oxalyl-coenzyme A reduction to glyoxylate is the preferred route of oxalate assimilation in Methylobacterium extorquens AM1.J Bacteriol. 2012 Jun;194(12):3144-55. doi: 10.1128/JB.00288-12. Epub 2012 Apr 6. J Bacteriol. 2012. PMID: 22493020 Free PMC article.
-
Biotechnological potential of the ethylmalonyl-CoA pathway.Appl Microbiol Biotechnol. 2011 Jan;89(1):17-25. doi: 10.1007/s00253-010-2873-z. Epub 2010 Sep 30. Appl Microbiol Biotechnol. 2011. PMID: 20882276 Review.
-
The Methylcitrate Cycle and Its Crosstalk with the Glyoxylate Cycle and Tricarboxylic Acid Cycle in Pathogenic Fungi.Molecules. 2023 Sep 17;28(18):6667. doi: 10.3390/molecules28186667. Molecules. 2023. PMID: 37764443 Free PMC article. Review.
Cited by
-
Coassimilation of organic substrates via the autotrophic 3-hydroxypropionate bi-cycle in Chloroflexus aurantiacus.Appl Environ Microbiol. 2011 Sep;77(17):6181-8. doi: 10.1128/AEM.00705-11. Epub 2011 Jul 15. Appl Environ Microbiol. 2011. PMID: 21764971 Free PMC article.
-
Streamlined pentafluorophenylpropyl column liquid chromatography-tandem quadrupole mass spectrometry and global (13)C-labeled internal standards improve performance for quantitative metabolomics in bacteria.J Chromatogr A. 2010 Nov 19;1217(47):7401-10. doi: 10.1016/j.chroma.2010.09.055. Epub 2010 Sep 29. J Chromatogr A. 2010. PMID: 20950815 Free PMC article.
-
Phosphoribosylpyrophosphate synthetase as a metabolic valve advances Methylobacterium/Methylorubrum phyllosphere colonization and plant growth.Nat Commun. 2024 Jul 16;15(1):5969. doi: 10.1038/s41467-024-50342-9. Nat Commun. 2024. PMID: 39013920 Free PMC article.
-
A novel engineered strain of Methylorubrum extorquens for methylotrophic production of glycolic acid.Microb Cell Fact. 2024 Dec 23;23(1):344. doi: 10.1186/s12934-024-02583-y. Microb Cell Fact. 2024. PMID: 39716233 Free PMC article.
-
Profiling of cytosolic and peroxisomal acetyl-CoA metabolism in Saccharomyces cerevisiae.PLoS One. 2012;7(8):e42475. doi: 10.1371/journal.pone.0042475. Epub 2012 Aug 2. PLoS One. 2012. PMID: 22876324 Free PMC article.
References
-
- Alber, B. E., R. Spanheimer, C. Ebenau-Jehle, and G. Fuchs. 2006. Study of an alternate glyoxylate cycle for acetate assimilation by Rhodobacter sphaeroides. Mol. Microbiol. 61:297-309. - PubMed
-
- Anthony, C. 1982. The Biochemistry of Methylotrophs. Academic Press, London, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

