Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling
- PMID: 20118270
- PMCID: PMC2850008
- DOI: 10.1104/pp.109.151647
Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling
Abstract
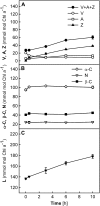
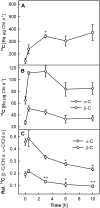
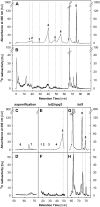
Carotenoid turnover was investigated in mature leaves of Arabidopsis (Arabidopsis thaliana) by 14CO2 pulse-chase labeling under control-light (CL; 130 micromol photons m(-2) s(-1)) and high-light (HL; 1,000 micromol photons m(-2) s(-1)) conditions. Following a 30-min 14CO2 administration, photosynthetically fixed 14C was quickly incorporated in beta-carotene (beta-C) and chlorophyll a (Chl a) in all samples during a chase of up to 10 h. In contrast, 14C was not detected in Chl b and xanthophylls, even when steady-state amounts of the xanthophyll-cycle pigments and lutein increased markedly, presumably by de novo synthesis, in CL-grown plants under HL. Different light conditions during the chase did not affect the 14C fractions incorporated in beta-C and Chl a, whereas long-term HL acclimation significantly enhanced 14C labeling of Chl a but not beta-C. Consequently, the maximal 14C signal ratio between beta-C and Chl a was much lower in HL-grown plants (1:10) than in CL-grown plants (1:4). In lut5 mutants, containing alpha-carotene (alpha-C) together with reduced amounts of beta-C, remarkably high 14C labeling was found for alpha-C while the labeling efficiency of Chl a was similar to that of wild-type plants. The maximum 14C ratios between carotenes and Chl a were 1:2 for alpha-C:Chl a and 1:5 for beta-C:Chl a in CL-grown lut5 plants, suggesting high turnover of alpha-C. The data demonstrate continuous synthesis and degradation of carotenes and Chl a in photosynthesizing leaves and indicate distinct acclimatory responses of their turnover to changing irradiance. In addition, the results are discussed in the context of photosystem II repair cycle and D1 protein turnover.
Figures




Similar articles
-
Altered turnover of β-carotene and Chl a in Arabidopsis leaves treated with lincomycin or norflurazon.Plant Cell Physiol. 2011 Jul;52(7):1193-203. doi: 10.1093/pcp/pcr069. Epub 2011 Jun 1. Plant Cell Physiol. 2011. PMID: 21632655
-
Acclimation of shade-tolerant and light-resistant Tradescantia species to growth light: chlorophyll a fluorescence, electron transport, and xanthophyll content.Photosynth Res. 2017 Sep;133(1-3):87-102. doi: 10.1007/s11120-017-0339-1. Epub 2017 Feb 8. Photosynth Res. 2017. PMID: 28176042
-
Lutein-mediated photoprotection of photosynthetic machinery in Arabidopsis thaliana exposed to chronic low ultraviolet-B radiation.J Plant Physiol. 2020 May;248:153160. doi: 10.1016/j.jplph.2020.153160. Epub 2020 Mar 30. J Plant Physiol. 2020. PMID: 32283468
-
Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence.J Photochem Photobiol B. 1996 Oct;36(1):3-15. doi: 10.1016/S1011-1344(96)07397-6. J Photochem Photobiol B. 1996. PMID: 8988608 Review.
-
Biosynthesis, accumulation and emission of carotenoids, alpha-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance.Photosynth Res. 2007 May;92(2):163-79. doi: 10.1007/s11120-007-9204-y. Epub 2007 Jul 17. Photosynth Res. 2007. PMID: 17634750 Review.
Cited by
-
Mutation in Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase Gene ZmCRD1 Causes Chlorophyll-Deficiency in Maize.Front Plant Sci. 2022 Jul 7;13:912215. doi: 10.3389/fpls.2022.912215. eCollection 2022. Front Plant Sci. 2022. PMID: 35873969 Free PMC article.
-
Root-zone glyphosate exposure adversely affects two ditch species.Biology (Basel). 2013 Dec 18;2(4):1488-96. doi: 10.3390/biology2041488. Biology (Basel). 2013. PMID: 24833234 Free PMC article.
-
Carotenoid metabolism and regulation in horticultural crops.Hortic Res. 2015 Aug 26;2:15036. doi: 10.1038/hortres.2015.36. eCollection 2015. Hortic Res. 2015. PMID: 26504578 Free PMC article. Review.
-
Emissions of carotenoid cleavage products upon heat shock and mechanical wounding from a foliose lichen.Environ Exp Bot. 2017 Jan;133:87-97. doi: 10.1016/j.envexpbot.2016.10.004. Epub 2016 Oct 7. Environ Exp Bot. 2017. PMID: 29416188 Free PMC article.
-
Carotenoid biosynthesis in Arabidopsis: a colorful pathway.Arabidopsis Book. 2012;10:e0158. doi: 10.1199/tab.0158. Epub 2012 Jan 19. Arabidopsis Book. 2012. PMID: 22582030 Free PMC article.
References
-
- Anderson JM, Chow WS, Goodchild DJ. (1988) Thylakoid membrane organisation in sun/shade acclimation. Aust J Plant Physiol 15: 11–26
-
- Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ. (2006a) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45: 982–993 - PubMed
-
- Auldridge ME, McCarty DR, Klee HJ. (2006b) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9: 315–321 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

