Control of alpha4beta7 integrin expression and CD4 T cell homing by the beta1 integrin subunit
- PMID: 20118278
- PMCID: PMC2824783
- DOI: 10.4049/jimmunol.0902407
Control of alpha4beta7 integrin expression and CD4 T cell homing by the beta1 integrin subunit
Abstract
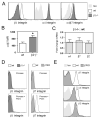
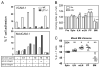
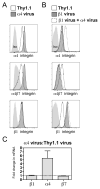
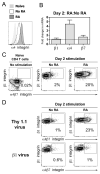
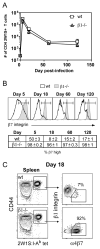
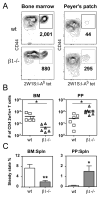
The alpha4beta7 integrin promotes homing of T cells to intestinal sites. The alpha4 integrin subunit that pairs with beta7 integrin can also pair with beta1 integrin. In this paper, we show that the preferential pairing of beta1 integrin with alpha4 integrin regulates the expression of alpha4beta7 on T cells. In the absence of beta1 integrin, naive mouse CD4 T cells have increased alpha4beta7 expression, resulting in increased adhesion to mucosal addressin cell adhesion molecule-1 and enhanced homing to Peyer's patches (PP). In a reciprocal manner, overexpression of beta1 integrin causes the loss of alpha4beta7 expression and decreased homing to PP. A similar upregulation of beta1 integrin and suppression of alpha4beta7 expression occurs rapidly after CD4 T cell activation. beta1 integrin thus dominates beta7 integrin for alpha4 integrin pairing, thereby controlling the abundance of unpaired alpha4 integrin. Increasing the abundance of alpha4 integrin relative to beta1 integrin is critical to retinoic acid-mediated expression of alpha4beta7 integrin during T cell activation. In the absence of beta1 integrin, endogenous Ag-specific CD4 T cells uniformly express high levels of alpha4beta7 after Listeria monocytogenes infection. The resulting beta1-deficient early memory T cells have decreased localization to the bone marrow and enhanced localization to PP after infection. Thus, the preferential association of beta1 integrin with alpha4 integrin suppresses alpha4beta7 integrin expression and regulates the localization of memory CD4 T cells.
Figures


Similar articles
-
Blockade of both L-selectin and alpha4 integrins abrogates naive CD4 cell trafficking and responses in gut-associated lymphoid organs.Int Immunol. 1998 Jul;10(7):961-8. doi: 10.1093/intimm/10.7.961. Int Immunol. 1998. PMID: 9701034
-
Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis.J Hepatol. 2020 Nov;73(5):1013-1022. doi: 10.1016/j.jhep.2020.05.047. Epub 2020 Jun 12. J Hepatol. 2020. PMID: 32540177 Free PMC article.
-
Preferential production of interferon-gamma by CD4+ T cells expressing the homing receptor integrin alpha4/beta7.Immunology. 2001 Jun;103(2):155-63. doi: 10.1046/j.0019-2805.2001.01234.x. Immunology. 2001. PMID: 11412302 Free PMC article.
-
Efficient lymphocyte migration across high endothelial venules of mouse Peyer's patches requires overlapping expression of L-selectin and beta7 integrin.J Immunol. 1998 Dec 15;161(12):6638-47. J Immunol. 1998. PMID: 9862692
-
β1 integrin is critical for the maintenance of antigen-specific CD4 T cells in the bone marrow but not long-term immunological memory.J Immunol. 2011 Apr 1;186(7):4019-26. doi: 10.4049/jimmunol.1003566. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357540 Free PMC article.
Cited by
-
Sustained immunotolerance in multiple sclerosis after stem cell transplant.Ann Clin Transl Neurol. 2022 Feb;9(2):206-220. doi: 10.1002/acn3.51510. Epub 2022 Feb 1. Ann Clin Transl Neurol. 2022. PMID: 35106961 Free PMC article. Clinical Trial.
-
Serosal Cavities Contain Two Populations of Innate-like integrin α4highCD4+ T Cells, Integrin α4β1+α6β1+α4β7- and α4β1+α6β1-α4β7+ Cells.Immune Netw. 2017 Dec;17(6):392-401. doi: 10.4110/in.2017.17.6.392. Epub 2017 Nov 24. Immune Netw. 2017. PMID: 29302252 Free PMC article.
-
CD161+ Tconv and CD161+ Treg Share a Transcriptional and Functional Phenotype despite Limited Overlap in TCRβ Repertoire.Front Immunol. 2017 Mar 6;8:103. doi: 10.3389/fimmu.2017.00103. eCollection 2017. Front Immunol. 2017. PMID: 28321213 Free PMC article.
-
Fat-soluble vitamins: updated review of their role and orchestration in human nutrition throughout life cycle with sex differences.Nutr Metab (Lond). 2022 Sep 5;19(1):60. doi: 10.1186/s12986-022-00696-y. Nutr Metab (Lond). 2022. PMID: 36064551 Free PMC article. Review.
-
Retinoic Acid as a Modulator of T Cell Immunity.Nutrients. 2016 Jun 13;8(6):349. doi: 10.3390/nu8060349. Nutrients. 2016. PMID: 27304965 Free PMC article. Review.
References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. - PubMed
-
- Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GE, Lazarovits AI, Buck D, Shaw S. Selective expression of integrin α4β7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J Immunol. 1993;151:717–729. - PubMed
-
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

