Entry of Spiroplasma citri into Circulifer haematoceps cells involves interaction between spiroplasma phosphoglycerate kinase and leafhopper actin
- PMID: 20118377
- PMCID: PMC2837992
- DOI: 10.1128/AEM.02384-09
Entry of Spiroplasma citri into Circulifer haematoceps cells involves interaction between spiroplasma phosphoglycerate kinase and leafhopper actin
Abstract
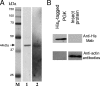
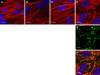
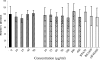
Transmission of the phytopathogenic mollicutes, spiroplasmas, and phytoplasmas by their insect vectors mainly depends on their ability to pass through gut cells, to multiply in various tissues, and to traverse the salivary gland cells. The passage of these different barriers suggests molecular interactions between the plant mollicute and the insect vector that regulate transmission. In the present study, we focused on the interaction between Spiroplasma citri and its leafhopper vector, Circulifer haematoceps. An in vitro protein overlay assay identified five significant binding activities between S. citri proteins and insect host proteins from salivary glands. One insect protein involved in one binding activity was identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as actin. Confocal microscopy observations of infected salivary glands revealed that spiroplasmas colocated with the host actin filaments. An S. citri actin-binding protein of 44 kDa was isolated by affinity chromatography and identified by LC-MS/MS as phosphoglycerate kinase (PGK). To investigate the role of the PGK-actin interaction, we performed competitive binding and internalization assays on leafhopper cultured cell lines (Ciha-1) in which His(6)-tagged PGK from S. citri or purified PGK from Saccharomyces cerevisiae was added prior to the addition of S. citri inoculum. The results suggested that exogenous PGK has no effect on spiroplasmal attachment to leafhopper cell surfaces but inhibits S. citri internalization, demonstrating that the process leading to internalization of S. citri in eukaryotic cells requires the presence of PGK. PGK, regardless of origin, reduced the entry of spiroplasmas into Ciha-1 cells in a dose-dependent manner.
Figures

Similar articles
-
Involvement of a minimal actin-binding region of Spiroplasma citri phosphoglycerate kinase in spiroplasma transmission by its leafhopper vector.PLoS One. 2011 Feb 22;6(2):e17357. doi: 10.1371/journal.pone.0017357. PLoS One. 2011. PMID: 21364953 Free PMC article.
-
Infection of the Circulifer haematoceps cell line Ciha-1 by Spiroplasma citri: the non-insect-transmissible strain 44 is impaired in invasion.Microbiology (Reading). 2010 Apr;156(Pt 4):1097-1107. doi: 10.1099/mic.0.035063-0. Epub 2009 Dec 17. Microbiology (Reading). 2010. PMID: 20019079
-
The repetitive domain of ScARP3d triggers entry of Spiroplasma citri into cultured cells of the vector Circulifer haematoceps.PLoS One. 2012;7(10):e48606. doi: 10.1371/journal.pone.0048606. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23119070 Free PMC article.
-
Spiroplasma citri, a plant pathogenic molligute: relationships with its two hosts, the plant and the leafhopper vector.Annu Rev Phytopathol. 2003;41:483-500. doi: 10.1146/annurev.phyto.41.052102.104034. Epub 2003 Apr 18. Annu Rev Phytopathol. 2003. PMID: 12730387 Review.
-
Spiroplasmas: infectious agents of plants, arthropods and vertebrates.Wien Klin Wochenschr. 1997 Aug 8;109(14-15):604-12. Wien Klin Wochenschr. 1997. PMID: 9286068 Review.
Cited by
-
Functional genomics of a Spiroplasma associated with the carmine cochineals Dactylopius coccus and Dactylopius opuntiae.BMC Genomics. 2021 Apr 6;22(1):240. doi: 10.1186/s12864-021-07540-2. BMC Genomics. 2021. PMID: 33823812 Free PMC article.
-
A New Perspective on the Co-Transmission of Plant Pathogens by Hemipterans.Microorganisms. 2023 Jan 7;11(1):156. doi: 10.3390/microorganisms11010156. Microorganisms. 2023. PMID: 36677448 Free PMC article. Review.
-
Sequences essential for transmission of Spiroplasma citri by its leafhopper vector, Circulifer haematoceps, revealed by plasmid curing and replacement based on incompatibility.Appl Environ Microbiol. 2010 May;76(10):3198-205. doi: 10.1128/AEM.00181-10. Epub 2010 Mar 19. Appl Environ Microbiol. 2010. PMID: 20305023 Free PMC article.
-
Phosphoglycerate kinase: structural aspects and functions, with special emphasis on the enzyme from Kinetoplastea.Open Biol. 2020 Nov;10(11):200302. doi: 10.1098/rsob.200302. Epub 2020 Nov 25. Open Biol. 2020. PMID: 33234025 Free PMC article. Review.
-
Role of the major antigenic membrane protein in phytoplasma transmission by two insect vector species.BMC Microbiol. 2015 Sep 30;15:193. doi: 10.1186/s12866-015-0522-5. BMC Microbiol. 2015. PMID: 26424332 Free PMC article.
References
-
- Alloush, H. M., J. L. Lopez-Ribot, B. J. Masten, and W. L. Chaffin. 1997. 3-Phosphoglycerate kinase: a glycolytic enzyme protein present in the cell wall of Candida albicans. Microbiology 143:321-330. - PubMed
-
- Ammar, E., and S. A. Hogenhout. 2005. Use of immunofluorescence confocal laser scanning microscopy to study distribution of the bacterium corn stunt spiroplasma in vector leafhoppers (Hemiptera: Cicadellidae) and in host plants. Ann. Entomol. Soc. Am. 98:820-826.
-
- Ammar, E.-D., D. Fulton, X. Bai, T. Meulia, and S. A. Hogenhout. 2004. An attachment tip and pili-like structures in insect- and plant-pathogenic spiroplasmas of the class Mollicutes. Arch. Microbiol. 181:97-105. - PubMed
-
- Andreev, O. A., and J. Borejdo. 1995. Binding of heavy-chain and essential light-chain 1 of S1 to actin depends on the degree of saturation of F-actin filaments with S1. Biochemistry 34:14829-14833. - PubMed
-
- Arnold, H., and D. Pette. 1968. Binding of glycolytic enzymes to structure proteins of the muscle. Eur. J. Biochem. 6:163-171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

