Entry of Spiroplasma citri into Circulifer haematoceps cells involves interaction between spiroplasma phosphoglycerate kinase and leafhopper actin
- PMID: 20118377
- PMCID: PMC2837992
- DOI: 10.1128/AEM.02384-09
Entry of Spiroplasma citri into Circulifer haematoceps cells involves interaction between spiroplasma phosphoglycerate kinase and leafhopper actin
Abstract
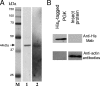
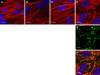
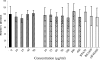
Transmission of the phytopathogenic mollicutes, spiroplasmas, and phytoplasmas by their insect vectors mainly depends on their ability to pass through gut cells, to multiply in various tissues, and to traverse the salivary gland cells. The passage of these different barriers suggests molecular interactions between the plant mollicute and the insect vector that regulate transmission. In the present study, we focused on the interaction between Spiroplasma citri and its leafhopper vector, Circulifer haematoceps. An in vitro protein overlay assay identified five significant binding activities between S. citri proteins and insect host proteins from salivary glands. One insect protein involved in one binding activity was identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as actin. Confocal microscopy observations of infected salivary glands revealed that spiroplasmas colocated with the host actin filaments. An S. citri actin-binding protein of 44 kDa was isolated by affinity chromatography and identified by LC-MS/MS as phosphoglycerate kinase (PGK). To investigate the role of the PGK-actin interaction, we performed competitive binding and internalization assays on leafhopper cultured cell lines (Ciha-1) in which His(6)-tagged PGK from S. citri or purified PGK from Saccharomyces cerevisiae was added prior to the addition of S. citri inoculum. The results suggested that exogenous PGK has no effect on spiroplasmal attachment to leafhopper cell surfaces but inhibits S. citri internalization, demonstrating that the process leading to internalization of S. citri in eukaryotic cells requires the presence of PGK. PGK, regardless of origin, reduced the entry of spiroplasmas into Ciha-1 cells in a dose-dependent manner.
Figures

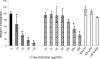
References
-
- Alloush, H. M., J. L. Lopez-Ribot, B. J. Masten, and W. L. Chaffin. 1997. 3-Phosphoglycerate kinase: a glycolytic enzyme protein present in the cell wall of Candida albicans. Microbiology 143:321-330. - PubMed
-
- Ammar, E., and S. A. Hogenhout. 2005. Use of immunofluorescence confocal laser scanning microscopy to study distribution of the bacterium corn stunt spiroplasma in vector leafhoppers (Hemiptera: Cicadellidae) and in host plants. Ann. Entomol. Soc. Am. 98:820-826.
-
- Ammar, E.-D., D. Fulton, X. Bai, T. Meulia, and S. A. Hogenhout. 2004. An attachment tip and pili-like structures in insect- and plant-pathogenic spiroplasmas of the class Mollicutes. Arch. Microbiol. 181:97-105. - PubMed
-
- Andreev, O. A., and J. Borejdo. 1995. Binding of heavy-chain and essential light-chain 1 of S1 to actin depends on the degree of saturation of F-actin filaments with S1. Biochemistry 34:14829-14833. - PubMed
-
- Arnold, H., and D. Pette. 1968. Binding of glycolytic enzymes to structure proteins of the muscle. Eur. J. Biochem. 6:163-171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

