Influenza vaccines: from surveillance through production to protection
- PMID: 20118381
- PMCID: PMC2843114
- DOI: 10.4065/mcp.2009.0615
Influenza vaccines: from surveillance through production to protection
Abstract
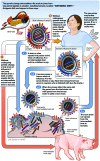
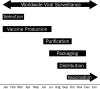
Influenza is an important contributor to population and individual morbidity and mortality. The current influenza pandemic with novel H1N1 has highlighted the need for health care professionals to better understand the processes involved in creating influenza vaccines, both for pandemic as well as for seasonal influenza. This review presents an overview of influenza-related topics to help meet this need and includes a discussion of the burden of disease, virology, epidemiology, viral surveillance, and vaccine strain selection. We then present an overview of influenza vaccine-related topics, including vaccine production, vaccine efficacy and effectiveness, influenza vaccine misperceptions, and populations that are recommended to receive vaccination. English-language articles in PubMed published between January 1, 1970, and October 7, 2009, were searched using key words human influenza, influenza vaccines, influenza A, and influenza B.
Figures

Similar articles
-
Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza.Vaccine. 2009 Aug 20;27(38):5184-6. doi: 10.1016/j.vaccine.2009.06.034. Epub 2009 Jun 27. Vaccine. 2009. PMID: 19563891
-
Seasonal influenza vaccine and protection against pandemic (H1N1) 2009-associated illness among US military personnel.PLoS One. 2010 May 19;5(5):e10722. doi: 10.1371/journal.pone.0010722. PLoS One. 2010. PMID: 20502705 Free PMC article.
-
[Principles and methods for vaccine epidemiology: evaluation of immunogenicity and effectiveness of pandemic H1N1 influenza vaccine].Nihon Eiseigaku Zasshi. 2013;68(3):153-60. doi: 10.1265/jjh.68.153. Nihon Eiseigaku Zasshi. 2013. PMID: 24077487 Review. Japanese.
-
Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine.Vaccine. 2012 Mar 2;30(11):1993-8. doi: 10.1016/j.vaccine.2011.12.098. Epub 2012 Jan 5. Vaccine. 2012. PMID: 22226861
-
Influenza vaccines: the good, the bad, and the eggs.Adv Virus Res. 2010;77:63-84. doi: 10.1016/B978-0-12-385034-8.00003-X. Adv Virus Res. 2010. PMID: 20951870 Review.
Cited by
-
Gaseous nitric oxide reduces influenza infectivity in vitro.Nitric Oxide. 2013 May 31;31:48-53. doi: 10.1016/j.niox.2013.03.007. Epub 2013 Apr 2. Nitric Oxide. 2013. PMID: 23562771 Free PMC article.
-
AS03-Adjuvanted, Very-Low-Dose Influenza Vaccines Induce Distinctive Immune Responses Compared to Unadjuvanted High-Dose Vaccines in BALB/c Mice.Front Immunol. 2015 Apr 29;6:207. doi: 10.3389/fimmu.2015.00207. eCollection 2015. Front Immunol. 2015. PMID: 25972874 Free PMC article.
-
The Role of Clinical Virology Laboratory and the Clinical Virology Laboratorian in Ensuring Effective Surveillance for Influenza and Other Respiratory Viruses: Points to Consider and Pitfalls to Avoid.Curr Treat Options Infect Dis. 2016;8(3):165-176. doi: 10.1007/s40506-016-0081-9. Epub 2016 Jul 5. Curr Treat Options Infect Dis. 2016. PMID: 32226325 Free PMC article. Review.
-
Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines.J Virol. 2011 Mar;85(6):3010-4. doi: 10.1128/JVI.01241-10. Epub 2010 Dec 29. J Virol. 2011. PMID: 21191017 Free PMC article.
-
Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus.J Immunol. 2011 Apr 1;186(7):4331-9. doi: 10.4049/jimmunol.1003057. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357542 Free PMC article.
References
-
- Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009 [published correction appears in MMWR Recomm Rep. 2009;58(32):896-897] MMWR Recomm Rep. 2009;58(RR-8):1-52 - PubMed
-
- Cho BH, Kolasa MS, Messonnier ML. Influenza vaccination coverage rate among high-risk children during the 2002-2003 influenza season. Am J Infect Control. 2008;36(8):582-587 - PubMed
-
- Loulergue P, Mir O, Alexandre J, Ropert S, Goldwasser F, Launay O. Low influenza vaccination rate among patients receiving chemotherapy for cancer [letter]. Ann Oncol. 2008;19(9):1658 - PubMed
-
- Nakamura MM, Lee GM. Influenza vaccination in adolescents with high-risk conditions. Pediatrics 2008;122(5):920-928 - PubMed
-
- Nowalk MP, Lin CJ, Zimmerman RK, et al. Self-reported influenza vaccination rates among health care workers in a large health system. Am J Infect Control. 2008;36(8):574-581 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

