Mesenchymal stem cells differentiate into renin-producing juxtaglomerular (JG)-like cells under the control of liver X receptor-alpha
- PMID: 20118482
- PMCID: PMC2852935
- DOI: 10.1074/jbc.M109.099671
Mesenchymal stem cells differentiate into renin-producing juxtaglomerular (JG)-like cells under the control of liver X receptor-alpha
Abstract
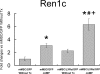
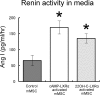
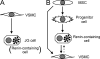
Renin is a key enzyme for cardiovascular and renal homeostasis and is produced by highly specialized endocrine cells in the kidney, known as juxtaglomerular (JG) cells. The nature and origin of these cells remain as mysteries. Previously, we have shown that the nuclear hormone receptor liver X receptor-alpha (LXRalpha) is a major transcriptional regulator of the expression of renin, c-myc, and other genes involved with growth/differentiation. In this study we test the hypothesis that LXRalpha plays an important role not only in renin expression but also in renin-containing cell differentiation, specifically from the mesenchymal stem cell (MSC), which may be the origin of the JG cell. Indeed, our data demonstrated that LXRalpha activation by its ligands or cAMP stimulated renin gene expression in both murine and human MSCs. Furthermore, sustained cAMP stimulation of murine MSCs overexpressing LXRalpha led to their differentiation into JG-like cells expressing renin and alpha-smooth muscle actin. These MSC-derived JG-like cells contained renin in secretory granules and released active renin in response to cAMP. In conclusion, the activation of LXRalpha stimulates renin expression and induces MSCs differentiation into renin-secreting, JG-like cells. Our results suggest that the MSC may be the origin of the juxtaglomerular cell and provide insight into novel understanding of pathophysiology of the renin-angiotensin system.
Figures
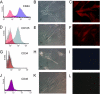
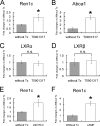
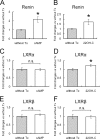
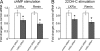


Similar articles
-
Adult renal mesenchymal stem cell-like cells contribute to juxtaglomerular cell recruitment.J Am Soc Nephrol. 2013 Jul;24(8):1263-73. doi: 10.1681/ASN.2012060596. Epub 2013 Jun 6. J Am Soc Nephrol. 2013. PMID: 23744888 Free PMC article.
-
Role of complement 3 in renin generation during the differentiation of mesenchymal stem cells to smooth muscle cells.Am J Physiol Cell Physiol. 2020 May 1;318(5):C981-C990. doi: 10.1152/ajpcell.00461.2019. Epub 2020 Mar 25. Am J Physiol Cell Physiol. 2020. PMID: 32208992
-
Sox6 as a new modulator of renin expression in the kidney.Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F285-F297. doi: 10.1152/ajprenal.00095.2019. Epub 2019 Nov 25. Am J Physiol Renal Physiol. 2020. PMID: 31760770 Free PMC article.
-
Regulation of renin secretion by renal juxtaglomerular cells.Pflugers Arch. 2013 Jan;465(1):25-37. doi: 10.1007/s00424-012-1126-7. Epub 2012 Jun 26. Pflugers Arch. 2013. PMID: 22733355 Review.
-
Transcriptional regulation of renin: an update.Hypertension. 2005 Jan;45(1):3-8. doi: 10.1161/01.HYP.0000149717.55920.45. Epub 2004 Nov 15. Hypertension. 2005. PMID: 15545507 Review.
Cited by
-
Deletion of angiotensin II type 2 receptor accelerates adipogenesis in murine mesenchymal stem cells via Wnt10b/beta-catenin signaling.Lab Invest. 2016 Aug;96(8):909-17. doi: 10.1038/labinvest.2016.66. Epub 2016 Jun 13. Lab Invest. 2016. PMID: 27295344 Free PMC article.
-
Recent advances involving the renin-angiotensin system.Exp Cell Res. 2012 May 15;318(9):1049-56. doi: 10.1016/j.yexcr.2012.02.023. Epub 2012 Mar 3. Exp Cell Res. 2012. PMID: 22410251 Free PMC article. Review.
-
The PPAR-gamma-binding sequence Pal3 is necessary for basal but dispensable for high-fat diet regulated human renin expression in the kidney.Pflugers Arch. 2017 Oct;469(10):1349-1357. doi: 10.1007/s00424-017-1994-y. Epub 2017 May 22. Pflugers Arch. 2017. PMID: 28534088
-
Contribution of the Local RAS to Hematopoietic Function: A Novel Therapeutic Target.Front Endocrinol (Lausanne). 2013 Oct 23;4:157. doi: 10.3389/fendo.2013.00157. Front Endocrinol (Lausanne). 2013. PMID: 24167502 Free PMC article. Review.
-
Human kidney pericytes produce renin.Kidney Int. 2016 Dec;90(6):1251-1261. doi: 10.1016/j.kint.2016.07.035. Epub 2016 Sep 24. Kidney Int. 2016. PMID: 27678158 Free PMC article.
References
-
- Ice K. S., Geary K. M., Gomez R. A., Johns D. W., Peach M. J., Carey R. M. (1988) Clin. Exp. Hypertens. A 10, 1169–1187 - PubMed
-
- Kon Y., Hashimoto Y., Murakami K., Sugimura M. (1992) Acta Anat. 144, 354–362 - PubMed
-
- Owen R. A., Molon-Noblot S., Hubert M. F., Kindt M. V., Keenan K. P., Eydelloth R. S. (1995) Toxicol. Pathol. 23, 606–619 - PubMed
-
- Sequeira López M. L., Pentz E. S., Nomasa T., Smithies O., Gomez R. A. (2004) Dev. Cell 6, 719–728 - PubMed
-
- Sequeira Lopez M. L., Pentz E. S., Robert B., Abrahamson D. R., Gomez R. A. (2001) Am. J. Physiol. Renal. Physiol. 281, F345–F356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

