Interdependence of amyloid formation in yeast: implications for polyglutamine disorders and biological functions
- PMID: 20118659
- PMCID: PMC2850420
- DOI: 10.4161/pri.4.1.11074
Interdependence of amyloid formation in yeast: implications for polyglutamine disorders and biological functions
Abstract
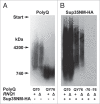
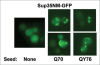
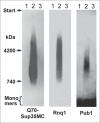
In eukaryotic cells amyloid aggregates may incorporate various functionally unrelated proteins. In mammalian diseases this may cause amyloid toxicity, while in yeast this could contribute to prion phenotypes. Insolubility of amyloids in the presence of strong ionic detergents, such as SDS or sarcosyl, allows discrimination between amorphous and amyloid aggregates. Here, we used this property of amyloids to study the interdependence of their formation in yeast. We observed that SDS-resistant polymers of proteins with extended polyglutamine domains caused the appearance of SDS or sarcosyl-insoluble polymers of three tested chromosomally-encoded Q/N-rich proteins, Sup35, Rnq1 and Pub1. These polymers were non-heritable, since they could not propagate in the absence of polyglutamine polymers. Sup35 prion polymers caused the appearance of non-heritable sarcosyl-resistant polymers of Pub1. Since eukaryotic genomes encode hundreds of proteins with long Q/N-rich regions, polymer interdependence suggests that conversion of a single protein into polymer form may significantly affect cell physiology by causing partial transfer of other Q/N-rich proteins into a non-functional polymer state.
Figures



Similar articles
-
Functional role of Tia1/Pub1 and Sup35 prion domains: directing protein synthesis machinery to the tubulin cytoskeleton.Mol Cell. 2014 Jul 17;55(2):305-18. doi: 10.1016/j.molcel.2014.05.027. Epub 2014 Jun 26. Mol Cell. 2014. PMID: 24981173 Free PMC article.
-
Appearance and propagation of polyglutamine-based amyloids in yeast: tyrosine residues enable polymer fragmentation.J Biol Chem. 2008 May 30;283(22):15185-92. doi: 10.1074/jbc.M802071200. Epub 2008 Apr 1. J Biol Chem. 2008. PMID: 18381282 Free PMC article.
-
Molecular basis for transmission barrier and interference between closely related prion proteins in yeast.J Biol Chem. 2011 May 6;286(18):15773-80. doi: 10.1074/jbc.M110.183889. Epub 2011 Mar 15. J Biol Chem. 2011. PMID: 21454674 Free PMC article.
-
Prion and nonprion amyloids: a comparison inspired by the yeast Sup35 protein.Prion. 2007 Jul-Sep;1(3):179-84. doi: 10.4161/pri.1.3.4840. Epub 2007 Jul 6. Prion. 2007. PMID: 19164899 Free PMC article. Review.
-
[Yeast as a model for studying the prion and amyloid occurrence].Ross Fiziol Zh Im I M Sechenova. 2004 May;90(5):645-57. Ross Fiziol Zh Im I M Sechenova. 2004. PMID: 15341089 Review. Russian.
Cited by
-
Functional role of Tia1/Pub1 and Sup35 prion domains: directing protein synthesis machinery to the tubulin cytoskeleton.Mol Cell. 2014 Jul 17;55(2):305-18. doi: 10.1016/j.molcel.2014.05.027. Epub 2014 Jun 26. Mol Cell. 2014. PMID: 24981173 Free PMC article.
-
Protein Co-Aggregation Related to Amyloids: Methods of Investigation, Diversity, and Classification.Int J Mol Sci. 2018 Aug 4;19(8):2292. doi: 10.3390/ijms19082292. Int J Mol Sci. 2018. PMID: 30081572 Free PMC article. Review.
-
Modeling Huntington disease in yeast: perspectives and future directions.Prion. 2011 Oct-Dec;5(4):269-76. doi: 10.4161/pri.18005. Epub 2011 Oct 1. Prion. 2011. PMID: 22052350 Free PMC article. Review.
-
Huntingtin Polyglutamine Fragments Are a Substrate for Hsp104 in Saccharomyces cerevisiae.Mol Cell Biol. 2021 Oct 26;41(11):e0012221. doi: 10.1128/MCB.00122-21. Epub 2021 Aug 23. Mol Cell Biol. 2021. PMID: 34424055 Free PMC article.
-
Amyloid-mediated sequestration of essential proteins contributes to mutant huntingtin toxicity in yeast.PLoS One. 2012;7(1):e29832. doi: 10.1371/journal.pone.0029832. Epub 2012 Jan 11. PLoS One. 2012. PMID: 22253794 Free PMC article.
References
-
- Gebbink MF, Claessen D, Bouma B, Dijkhuizen L, Wösten HA. Amyloids—a functional coat for microorganisms. Nat Rev Microbiol. 2005;3:333–341. - PubMed
-
- Mironova LN, Goginashvili AI, Ter-Avanesyan MD. Biological functions of amyloids: facts and hypotheses. Mol Biol. 2008;42:710–719. - PubMed
-
- Vishnevskaya AB, Kushnirov VV, Ter-Avanesyan MD. Neurodegenerative amyloidoses: yeast model. Mol Biol. 2007;41:346–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
