Nuclear factor (erythroid-derived 2)-like 2 regulates drug resistance in pancreatic cancer cells
- PMID: 20118824
- PMCID: PMC3506252
- DOI: 10.1097/MPA.0b013e3181c31314
Nuclear factor (erythroid-derived 2)-like 2 regulates drug resistance in pancreatic cancer cells
Abstract
Objective: To investigate the molecular basis of drug resistance in pancreatic cancer.
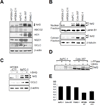
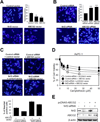
Methods: The expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) levels in pancreatic cancer tissues and cell lines was analyzed. Clinical relevance between Nrf2 activation and drug resistance was demonstrated by measuring cell viability after Nrf2 and adenosine 5'-triphosphate-binding cassette, subfamily G member 2 (ABCG2) regulation by overexpression or knock-down of these genes. Activity of ABCG2 was measured by Hoechst 33342 staining.
Results: Abnormally elevated Nrf2 protein levels were observed in pancreatic cancer tissues and cell lines relative to normal pancreatic tissues. Increasing Nrf2 protein levels either by overexpression of exogenous Nrf2 or by activating endogenous Nrf2 resulted in increased drug resistance. Conversely, a reduction in endogenous Nrf2 protein levels or inactivation of endogenous Nrf2 resulted in decreased drug resistance. These changes in drug resistance or sensitivity were also positively correlated to the expression levels of Nrf2 downstream genes. Similarly, the expression of ABCG2 was correlated with drug resistance.
Conclusions: Because the intrinsic drug resistance of pancreatic cancers is, in part, due to abnormally elevated Nrf2 protein levels, further research on regulating Nrf2 activity may result in the development of novel pancreatic cancer therapies.
Figures




Similar articles
-
NRF2 Knockdown Resensitizes 5-Fluorouracil-Resistant Pancreatic Cancer Cells by Suppressing HO-1 and ABCG2 Expression.Int J Mol Sci. 2020 Jun 30;21(13):4646. doi: 10.3390/ijms21134646. Int J Mol Sci. 2020. PMID: 32629871 Free PMC article.
-
Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype.Mol Cancer Ther. 2010 Aug;9(8):2365-76. doi: 10.1158/1535-7163.MCT-10-0108. Epub 2010 Aug 3. Mol Cancer Ther. 2010. PMID: 20682644 Free PMC article.
-
Elevated Expression of Nrf-2 and ABCG2 Involved in Multi-drug Resistance of Lung Cancer SP Cells.Drug Res (Stuttg). 2015 Oct;65(10):526-31. doi: 10.1055/s-0034-1390458. Epub 2014 Nov 4. Drug Res (Stuttg). 2015. PMID: 25368906
-
Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2).Oncogene. 2003 Oct 20;22(47):7340-58. doi: 10.1038/sj.onc.1206938. Oncogene. 2003. PMID: 14576842 Review.
-
Role of Nrf2 in cancer photodynamic therapy: regulation of human ABC transporter ABCG2.J Pharm Sci. 2013 Sep;102(9):3058-69. doi: 10.1002/jps.23563. Epub 2013 May 6. J Pharm Sci. 2013. PMID: 23650051 Review.
Cited by
-
HEATR1 deficiency promotes pancreatic cancer proliferation and gemcitabine resistance by up-regulating Nrf2 signaling.Redox Biol. 2020 Jan;29:101390. doi: 10.1016/j.redox.2019.101390. Epub 2019 Nov 20. Redox Biol. 2020. PMID: 31785531 Free PMC article.
-
Dual roles and therapeutic potential of Keap1-Nrf2 pathway in pancreatic cancer: a systematic review.Cell Commun Signal. 2019 Sep 11;17(1):121. doi: 10.1186/s12964-019-0435-2. Cell Commun Signal. 2019. PMID: 31511020 Free PMC article.
-
lncRNA SLC7A11-AS1 Promotes Chemoresistance by Blocking SCFβ-TRCP-Mediated Degradation of NRF2 in Pancreatic Cancer.Mol Ther Nucleic Acids. 2020 Mar 6;19:974-985. doi: 10.1016/j.omtn.2019.11.035. Epub 2020 Jan 11. Mol Ther Nucleic Acids. 2020. PMID: 32036249 Free PMC article.
-
HER2 confers drug resistance of human breast cancer cells through activation of NRF2 by direct interaction.Sci Rep. 2014 Dec 3;4:7201. doi: 10.1038/srep07201. Sci Rep. 2014. PMID: 25467193 Free PMC article.
-
Wogonin reversed resistant human myelogenous leukemia cells via inhibiting Nrf2 signaling by Stat3/NF-κB inactivation.Sci Rep. 2017 Feb 2;7:39950. doi: 10.1038/srep39950. Sci Rep. 2017. Retraction in: Sci Rep. 2022 May 30;12(1):9007. doi: 10.1038/s41598-022-13462-0. PMID: 28150717 Free PMC article. Retracted.
References
-
- Jemal A, Murray T, Ward E, et al. Cancer Statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Kim R, Saif MW. Is there an optimal neoadjuvant therapy for locally advanced pancreatic cancer? JOP. 2007;8:279–288. - PubMed
-
- Kang SP, Saif MW. Pharmacogenomics and pancreatic cancer treatment. Optimizing current therapy and individualizing future therapy. J of Pancreas. 2008;9:251–266. - PubMed
-
- Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. - PubMed
-
- Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

