Dichotomy of functional organization in the mouse auditory cortex
- PMID: 20118924
- PMCID: PMC2866453
- DOI: 10.1038/nn.2490
Dichotomy of functional organization in the mouse auditory cortex
Abstract
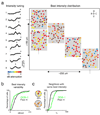
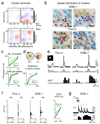
The sensory areas of the cerebral cortex possess multiple topographic representations of sensory dimensions. The gradient of frequency selectivity (tonotopy) is the dominant organizational feature in the primary auditory cortex, whereas other feature-based organizations are less well established. We probed the topographic organization of the mouse auditory cortex at the single-cell level using in vivo two-photon Ca(2+) imaging. Tonotopy was present on a large scale but was fractured on a fine scale. Intensity tuning, which is important in level-invariant representation, was observed in individual cells, but was not topographically organized. The presence or near absence of putative subthreshold responses revealed a dichotomy in topographic organization. Inclusion of subthreshold responses revealed a topographic clustering of neurons with similar response properties, whereas such clustering was absent in supra-threshold responses. This dichotomy indicates that groups of nearby neurons with locally shared inputs can perform independent parallel computations in the auditory cortex.
Figures


Comment in
-
Changing tune in auditory cortex.Nat Neurosci. 2010 Mar;13(3):271-3. doi: 10.1038/nn0310-271. Nat Neurosci. 2010. PMID: 20177415 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

