Factors influencing time course of pain after depot oil intramuscular injection of testosterone undecanoate
- PMID: 20118950
- PMCID: PMC3739103
- DOI: 10.1038/aja.2010.1
Factors influencing time course of pain after depot oil intramuscular injection of testosterone undecanoate
Abstract
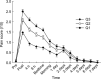
Pain following depot intramuscular (IM) injection of oil vehicle-based drugs has been little studied. This study aimed to determine prospectively the prevalence, determinants, severity and functional consequences of pain during the week after IM injection of 1 000 mg testosterone undecanoate (TU) in a 4-mL castor oil vehicle. Androgen-deficient men receiving regular T replacement therapy at an academic andrology clinic were recruited to report pain scores using a coloured visual linear analogue scale at seven times over the first day and daily for a week after a deep IM gluteal injection. The time course and covariables influencing pain scores were analysed by mixed model analysis of variance (ANOVA). Following 168 injections in 125 men, pain was reported by 80% of men, peaking immediately after injection, reaching only moderate severity, lasting 1-2 days and returning to baseline by day 4. The pain required little analgesic use and produced minimal interference in daily activities. The time course of pain scores was reproducible in the 43 men who underwent two consecutive injections. Pain was more severe in men who had an earlier painful injection, but less severe in older and more obese men. There were negligible differences in post-injection pain experience between experienced nurses administering injections. Deep IM gluteal injection of depot TU in 4-mL castor oil is well tolerated and post-injection pain is influenced by earlier painful injection experience, as well as age and obesity.
Figures



Similar articles
-
Pharmacokinetics and Acceptability of Subcutaneous Injection of Testosterone Undecanoate.J Endocr Soc. 2019 Jun 28;3(8):1531-1540. doi: 10.1210/js.2019-00134. eCollection 2019 Aug 1. J Endocr Soc. 2019. PMID: 31384715 Free PMC article.
-
Tolerability of intramuscular injections of testosterone ester in oil vehicle.Hum Reprod. 1995 Apr;10(4):862-5. doi: 10.1093/oxfordjournals.humrep.a136051. Hum Reprod. 1995. PMID: 7650133 Clinical Trial.
-
Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies.Eur J Endocrinol. 1999 May;140(5):414-9. doi: 10.1530/eje.0.1400414. Eur J Endocrinol. 1999. PMID: 10229906 Clinical Trial.
-
Occurrence of pulmonary oil microembolism (POME) with intramuscular testosterone undecanoate injection: literature review.Int J Impot Res. 2023 Aug;35(5):439-446. doi: 10.1038/s41443-022-00585-1. Epub 2022 May 24. Int J Impot Res. 2023. PMID: 35610506 Review.
-
Testosterone depot injection in male hypogonadism: a critical appraisal.Clin Interv Aging. 2007;2(4):577-90. Clin Interv Aging. 2007. PMID: 18225458 Free PMC article. Review.
Cited by
-
Novel double-layer Silastic testicular prosthesis with controlled release of testosterone in vitro, and its effects on castrated rats.Asian J Androl. 2017 Jul-Aug;19(4):433-438. doi: 10.4103/1008-682X.175786. Asian J Androl. 2017. PMID: 27174160 Free PMC article.
-
Local Tissue Response to Subcutaneous Administration of Ceftriaxone in an Animal Model.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e02090-19. doi: 10.1128/AAC.02090-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31871078 Free PMC article.
-
Occurrence of Pulmonary Oil Microembolism After Testosterone Undecanoate Injection: A Postmarketing Safety Analysis.Sex Med. 2020 Jun;8(2):237-242. doi: 10.1016/j.esxm.2020.01.009. Epub 2020 Mar 14. Sex Med. 2020. PMID: 32184081 Free PMC article.
-
Pharmacokinetics and Acceptability of Subcutaneous Injection of Testosterone Undecanoate.J Endocr Soc. 2019 Jun 28;3(8):1531-1540. doi: 10.1210/js.2019-00134. eCollection 2019 Aug 1. J Endocr Soc. 2019. PMID: 31384715 Free PMC article.
-
Testosterone Therapy With Subcutaneous Injections: A Safe, Practical, and Reasonable Option.J Clin Endocrinol Metab. 2022 Feb 17;107(3):614-626. doi: 10.1210/clinem/dgab772. J Clin Endocrinol Metab. 2022. PMID: 34698352 Free PMC article.
References
-
- Greenblatt DJ, Koch-Weser J. Intramuscular injection of drugs. N Engl J Med. 1976;295:542–6. - PubMed
-
- Junkman K. Long-acting steroids in reproduction. Recent Prog Horm Res. 1957;13:380–419. - PubMed
-
- Altamura AC, Sassella F, Santini A, Montresor C, Fumagalli S, et al. Intramuscular preparations of antipsychotics: uses and relevance in clinical practice. Drugs. 2003;63:493–512. - PubMed
-
- Bhanji NH, Chouinard G, Margolese HC. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Eur Neuropsychopharmacol. 2004;14:87–92. - PubMed
-
- Handelsman DJ.Androgen Physiology, Pharmacology and AbuseIn: DeGroot LJ, Jameson JL, editors. Endocrinology6th edn. Philadelphia: Elsevier Saunders; 2010. in press).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

